到 2027 年北美药物警戒和药物安全软件市场预测 - 按软件类型划分的 COVID-19 影响和区域分析(不良事件报告软件、药物安全审计软件、问题跟踪软件和完全集成的软件);交付模式(本地和基于云);最终用户(制药和生物技术公司、合同研究组织 (CRO) 和业务流程外包 (BPO) 公司)
No. of Pages: 121 | Report Code: TIPRE00022774 | Category: Technology, Media and Telecommunications
No. of Pages: 121 | Report Code: TIPRE00022774 | Category: Technology, Media and Telecommunications
药物警戒(PV)通过评估、监测和发现药物相互作用及其作用,在医疗保健系统中发挥着重要作用。对人体的影响。药物警戒帮助公司在试验阶段和上市后期间监测任何药物不良反应事件。
因此,药物警戒的全球化预计将对药物警戒和药物安全软件产生巨大需求。未来几年,预计这将进一步推动药物警戒和药物安全软件市场的发展。
自 COVID-19 爆发以来,北美的 COVID-19 数量不断增加。正在采取多项措施来抑制疾病并防止传播;然而,过多的新冠病例导致医生预约被取消,由于长期封锁,对选择性药物警戒和药物安全软件的需求减少。在美国,由于受污染的患者数量不断增加,医疗保健从业者和领先组织正在分散医疗服务从研发到初级保健的流程,从而减慢了创新进程。此外,许多卫生组织,即美国食品和药物管理局(FDA)正在评估这一流行病的影响,以及未来几年如何处理临床试验和药物警戒计划。这种迅速变化的情况意味着申办者需要更加谨慎和勤奋地评估大流行的后果和当局期望的变化,以尽量减少对安全报告的影响并确保患者安全。要求更新指南和快速实施监管变革的价值比以往任何时候都更大,因为它可以减轻对临床试验的干扰。为了成功处理高度复杂的情况,可能需要经过验证的全面安全报告解决方案。
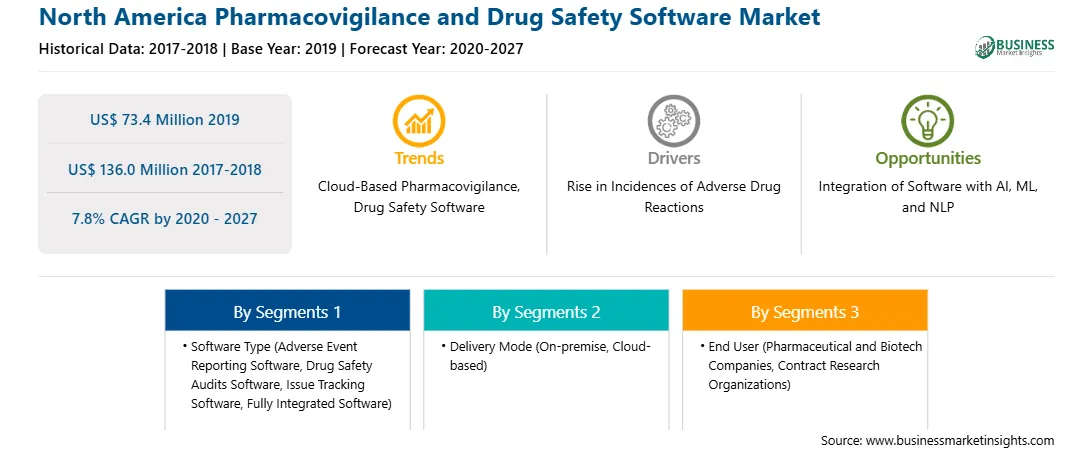
北美药物警戒和药物安全软件市场预计将从2019年的7340万美元增长到136.0美元到 2027 年将达到 100 万;预计 2020 年至 2027 年复合年增长率为 7.8%。人工智能 (AI) 有望成为药物警戒领域的一场革命。它将有助于降低病例报告成本并提高数据质量。这将使专家能够专注于其他增值流程,包括药物安全中的信号检测、药物警戒分析和效益风险评估。人工智能(AI),系统中可以分析源文件并选择适当的内容,并根据机器学习执行病例接收、严重性评估、病例处理以及医疗审查和病例监测。单调和常规的手动任务(例如不良事件案例报告)可以由人工智能以非常复杂和流畅的方式自动化和处理。通过将人工智能应用于不良事件案例处理并让药物警戒资源专注于战略活动,生命科学公司将获得更好的结果。这是因为安全单元可以通过触手可及的可靠数据以更智能、更快速的方式工作。因此,人工智能在药物警戒领域的发展可能会推动市场的增长。
< strong>主要细分市场
从软件类型来看,不良事件报告软件细分市场占据北美药物警戒和药物安全软件市场最大份额2019年,从交付模式来看,内部部署细分市场在2019年药物警戒和药物安全软件市场中占据了更大的市场份额。从最终用户来看,合同研究组织细分市场在药物警戒和药物安全软件市场中占据了更大的市场份额。 2019年药品安全软件市场。
准备本北美药物警戒和药物安全软件市场报告时提到的一些主要一手和二手来源是公司网站、年度报告、财务报告、国家政府文件、统计数据库等。报告中列出的主要公司有 Veeva Systems
按软件类型
按交付模式< /p>
最终用户 span>
< /p>
按国家/地区
Strategic insights for North America Pharmacovigilance and Drug Safety Software involve closely monitoring industry trends, consumer behaviours, and competitor actions to identify opportunities for growth. By leveraging data analytics, businesses can anticipate market shifts and make informed decisions that align with evolving customer needs. Understanding these dynamics helps companies adjust their strategies proactively, enhance customer engagement, and strengthen their competitive edge. Building strong relationships with stakeholders and staying agile in response to changes ensures long-term success in any market.
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 73.4 Million |
| Market Size by 2027 | US$ 136.0 Million |
| Global CAGR (2020 - 2027) | 7.8% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By 软件类型
|
| Regions and Countries Covered | 北美
|
| Market leaders and key company profiles |
The regional scope of North America Pharmacovigilance and Drug Safety Software refers to the geographical area in which a business operates and competes. Understanding regional nuances, such as local consumer preferences, economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved regions or adapting their offerings to meet regional demands. A clear regional focus allows for more effective resource allocation, targeted marketing, and better positioning against local competitors, ultimately driving growth in those specific areas.
The North America Pharmacovigilance and Drug Safety Software Market is valued at US$ 73.4 Million in 2019, it is projected to reach US$ 136.0 Million by 2027.
As per our report North America Pharmacovigilance and Drug Safety Software Market, the market size is valued at US$ 73.4 Million in 2019, projecting it to reach US$ 136.0 Million by 2027. This translates to a CAGR of approximately 7.8% during the forecast period.
The North America Pharmacovigilance and Drug Safety Software Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Pharmacovigilance and Drug Safety Software Market report:
The North America Pharmacovigilance and Drug Safety Software Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Pharmacovigilance and Drug Safety Software Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Pharmacovigilance and Drug Safety Software Market value chain can benefit from the information contained in a comprehensive market report.