欧洲镍钛诺医疗器械市场预测至 2028 年 - COVID-19 影响和区域分析(按产品(镍钛诺支架、镍钛诺导丝、镍钛诺过滤器、镍钛诺篮、镍钛诺导管等)和应用(骨科、血管、牙科和胃肠病学)
No. of Pages: 139 | Report Code: TIPRE00023711 | Category: Life Sciences
No. of Pages: 139 | Report Code: TIPRE00023711 | Category: Life Sciences
镍钛诺是指镍和钛的合金,它正迅速成为各种成分的首选金属医疗保健行业的医疗设备。镍钛诺广泛应用于自膨胀移植物、篮子、过滤器、移植物支撑系统等。镍钛诺合金以其超弹性和热形状记忆而闻名。
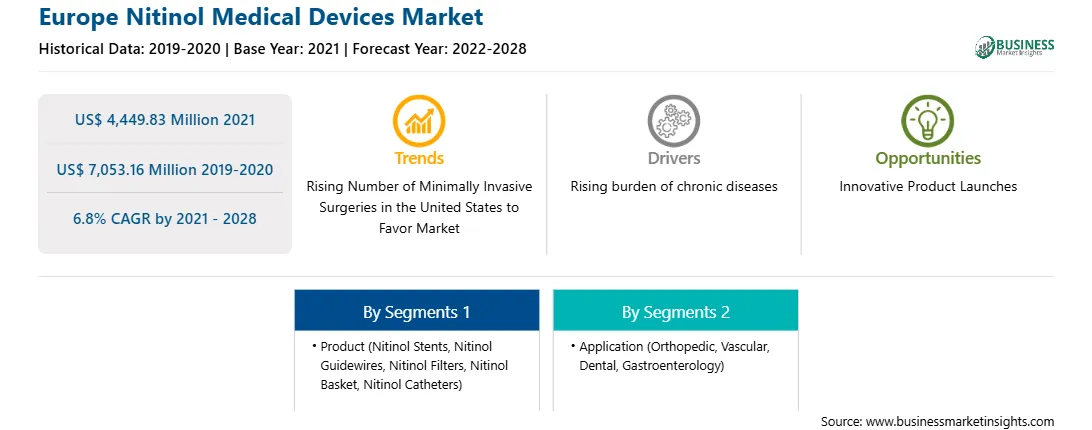
欧洲镍钛诺医疗器械市场预计将从2021年的444983万美元增至2028年的705316万美元;预计 2021 年至 2028 年复合年增长率为 6.8%。市场的增长归因于微创手术 (MIS) 的采用不断增加,以及监管机构制定的镍钛诺器械生物相容性监管指南正在推动市场发展。然而,包括连接和组装、电镀和涂层、机械加工、冲压和成型在内的制造挑战可能会阻碍市场的增长。
微创技术的使用外科手术已经发展了医学科学中外科手术的范式。 MIS 的优点,如切口更小、疼痛减轻、并发症更少、失血量最少和住院时间更短,极大地影响了 MIS 的采用。 MIS 的日益普及主要得益于 MIS 的优势,它同样有助于利用专门用于机器人手术和腹腔镜手术的镍钛合金医疗设备的增长。因此,通过 MIS 进行的程序数量的增加可能会在预测期内推动市场。然而,由于存在一些制造挑战,监管机构正在努力提高镍钛诺设备的生物相容性和稳定性,以实现更广泛的医疗应用。进行各种评估以建立镍钛合金产品的统一制造指南。 2020 年 10 月,美国食品和药物管理局 (FDA) 等监管机构发布了与镍钛诺器械与皮肤生物相容性及其技术细节的上市前提交相关的新指南。因此,指南的发布预计将增强镍钛诺在开发更广泛应用的医疗设备中的使用,从而在预测期内为制造商提供重要的增长机会。
COVID-19 大流行对欧洲卫生经济产生了重大负面影响。欧洲地区的卫生服务高度优先为受 COVID-19 影响的患者提供服务,常规卫生保健服务仍处于暂停状态。因此,它影响了镍钛诺医疗器械相关市场及其在该地区的可靠创收。欧洲国家的封锁广泛影响了区内及区外的企业流动。与行业相关的各种会议正在通过网络研讨会举行。因此,预计未来几年对镍钛诺医疗器械市场的负面影响可能会持续。
欧洲镍钛诺医疗器械市场按产品细分为镍钛诺支架、镍钛诺导丝、镍钛诺过滤器、镍钛诺篮、镍钛诺导管等。 2020 年,按产品划分,镍钛诺支架细分市场占据最大市场份额。预计同一细分市场在预测期内将以显着的复合年增长率增长。
欧洲镍钛诺医疗器械市场按应用细分为骨科、血管、牙科和胃肠病学。 2020 年,按应用划分,血管细分市场占据最大市场份额。此外,类似的细分市场预计在未来几年将以最快的速度增长。
与本欧洲镍钛诺医疗器械市场报告相关的一些主要和二手来源是世界卫生组织 (WHO)、西班牙国家卫生系统 (NHS) 和英国肾脏保健中心。
购买报告的理由
按产品
通过应用
Strategic insights for Europe Nitinol Medical Devices involve closely monitoring industry trends, consumer behaviours, and competitor actions to identify opportunities for growth. By leveraging data analytics, businesses can anticipate market shifts and make informed decisions that align with evolving customer needs. Understanding these dynamics helps companies adjust their strategies proactively, enhance customer engagement, and strengthen their competitive edge. Building strong relationships with stakeholders and staying agile in response to changes ensures long-term success in any market.
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 4,449.83 Million |
| Market Size by 2028 | US$ 7,053.16 Million |
| Global CAGR (2021 - 2028) | 6.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By 产品
|
| Regions and Countries Covered | 欧洲
|
| Market leaders and key company profiles |
The regional scope of Europe Nitinol Medical Devices refers to the geographical area in which a business operates and competes. Understanding regional nuances, such as local consumer preferences, economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved regions or adapting their offerings to meet regional demands. A clear regional focus allows for more effective resource allocation, targeted marketing, and better positioning against local competitors, ultimately driving growth in those specific areas.
The Europe Nitinol Medical Devices Market is valued at US$ 4,449.83 Million in 2021, it is projected to reach US$ 7,053.16 Million by 2028.
As per our report Europe Nitinol Medical Devices Market, the market size is valued at US$ 4,449.83 Million in 2021, projecting it to reach US$ 7,053.16 Million by 2028. This translates to a CAGR of approximately 6.8% during the forecast period.
The Europe Nitinol Medical Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Nitinol Medical Devices Market report:
The Europe Nitinol Medical Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Nitinol Medical Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Nitinol Medical Devices Market value chain can benefit from the information contained in a comprehensive market report.