欧洲医疗器械警戒软件市场规模和预测(2021 - 2031 年)、区域份额、趋势和增长机会分析报告范围:按应用(诊断、治疗、外科、研究等)、部署模式(云和本地)、最终用途垂直行业 [临床研究组织 (CRO)、业务流程外包 (BPO)、原始设备制造商 (OEM) 等] 和国家/地区
No. of Pages: 101 | Report Code: BMIRE00030961 | Category: Technology, Media and Telecommunications
No. of Pages: 101 | Report Code: BMIRE00030961 | Category: Technology, Media and Telecommunications
Strategic insights for Europe Medical Device Vigilance Software involve closely monitoring industry trends, consumer behaviours, and competitor actions to identify opportunities for growth. By leveraging data analytics, businesses can anticipate market shifts and make informed decisions that align with evolving customer needs. Understanding these dynamics helps companies adjust their strategies proactively, enhance customer engagement, and strengthen their competitive edge. Building strong relationships with stakeholders and staying agile in response to changes ensures long-term success in any market.
| Report Attribute | Details |
|---|---|
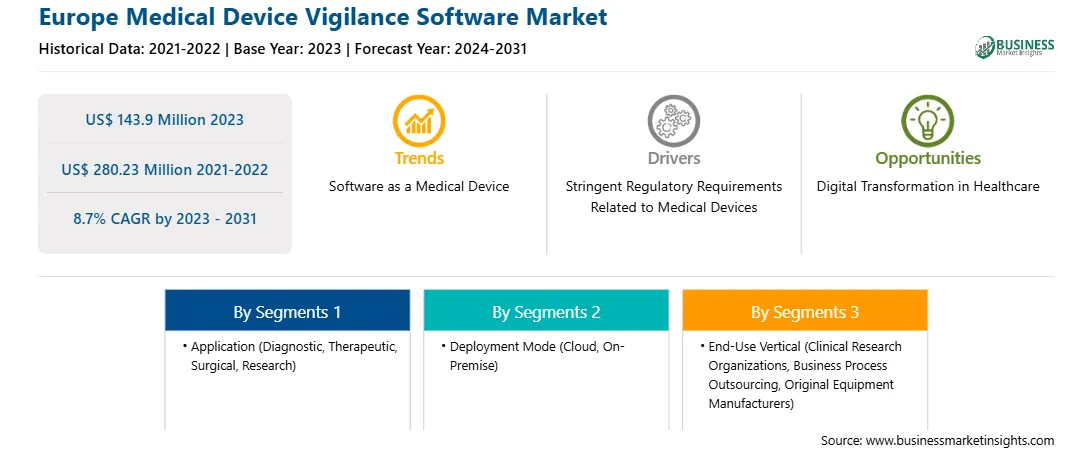
| Market size in 2023 | US$ 143.9 Million |
| Market Size by 2031 | US$ 280.23 Million |
| Global CAGR (2023 - 2031) | 8.7% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By 应用
|
| Regions and Countries Covered | 欧洲
|
| Market leaders and key company profiles |
The regional scope of Europe Medical Device Vigilance Software refers to the geographical area in which a business operates and competes. Understanding regional nuances, such as local consumer preferences, economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved regions or adapting their offerings to meet regional demands. A clear regional focus allows for more effective resource allocation, targeted marketing, and better positioning against local competitors, ultimately driving growth in those specific areas.
The Europe Medical Device Vigilance Software Market is valued at US$ 143.9 Million in 2023, it is projected to reach US$ 280.23 Million by 2031.
As per our report Europe Medical Device Vigilance Software Market, the market size is valued at US$ 143.9 Million in 2023, projecting it to reach US$ 280.23 Million by 2031. This translates to a CAGR of approximately 8.7% during the forecast period.
The Europe Medical Device Vigilance Software Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Medical Device Vigilance Software Market report:
The Europe Medical Device Vigilance Software Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Medical Device Vigilance Software Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Medical Device Vigilance Software Market value chain can benefit from the information contained in a comprehensive market report.