亚太细胞治疗市场预测至 2027 年 - 按治疗类型(同种异体和自体)、产品(消耗品、设备、系统和软件)、技术(病毒载体技术、基因组编辑技术、体细胞技术)进行的 COVID-19 影响和区域分析、细胞永生化技术、细胞可塑性技术和三维技术)、应用(肿瘤学、心血管、骨科、伤口管理和其他应用)和最终用户(研究机构、医院等)和国家/地区
No. of Pages: 108 | Report Code: TIPRE00019148 | Category: Life Sciences
No. of Pages: 108 | Report Code: TIPRE00019148 | Category: Life Sciences
本研究报告提供了对亚太细胞治疗市场的见解。细胞疗法是将活的完整细胞注射、植入或移植到患者体内的过程。这项技术依赖于用健康的功能细胞替代功能失调的细胞。主要用于此类先进疗法的细胞是干细胞,因为它们能够分化成修复受损或有缺陷的组织或细胞所需的特定细胞。此外,细胞疗法在再生医学的开发中也有应用
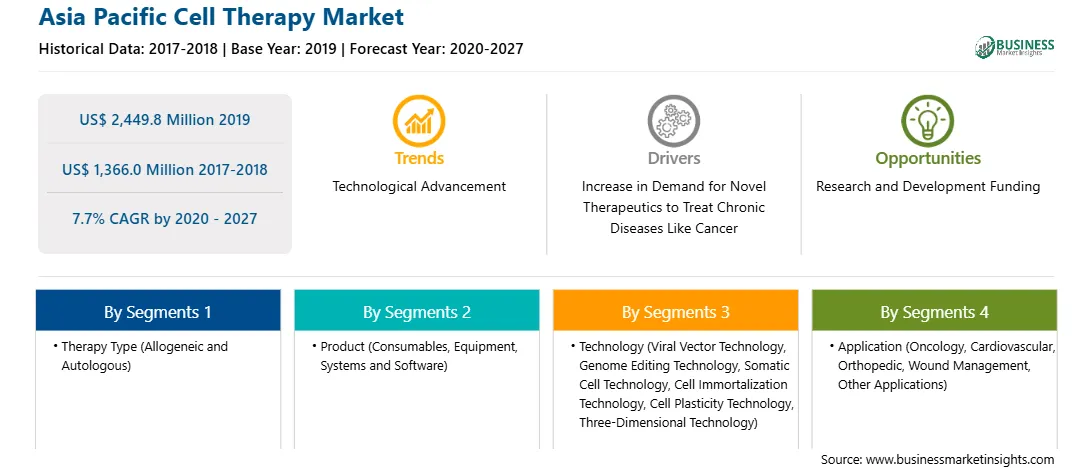
亚太地区细胞疗法预计将从2019年的24.498亿美元增至2027年的13.66亿美元。市场预计 2020 年至 2027 年复合年增长率为 7.7%。生物技术的进步导致针对多种适应症采用个性化治疗。干细胞疗法被用于治疗慢性疾病,例如癌症、神经系统疾病和遗传性疾病。再者,细胞疗法的优势,如靶向治疗、恢复更快、更有效、副作用减少等;促进各种产品的采用。在北美,由于 FDA 批准的产品的可用性,细胞疗法被广泛采用。例如,2020 年 4 月,FDA 向诺华公司的 Kymriah 授予再生医学先进疗法称号,用于治疗成人难治性 (r/r) 滤泡性淋巴瘤 (FL)。 2020年7月,FDA批准了CAR T细胞疗法brexucabtagene autoleucel(Tecartus)用于治疗套细胞淋巴瘤患者。这是 FDA 批准的首个治疗套细胞淋巴瘤的 CAR T 细胞疗法,并通过加速审批途径获得批准。 Tecartus 还获得了孤儿药称号,该称号鼓励罕见疾病药物的开发。其他获批的癌症 CAR-T 细胞疗法包括用于治疗急性淋巴细胞白血病的 Kymriah 和用于治疗弥漫性大 B 细胞淋巴瘤的 Yescarta。除此之外,全球有超过 250 项研究 CAR T 细胞疗法的临床试验,更多的细胞疗法正在开发中,其潜在适应症包括胰腺癌、自身免疫性疾病、阿尔茨海默氏症和帕金森氏症。预计这将推动细胞治疗市场的发展。
世界卫生组织 (WHO)、美国食品和药物管理局 (FDA) 是亚太细胞疗法的一些主要主要和次要来源报告中包含的市场。
Strategic insights for Asia Pacific Cell Therapy involve closely monitoring industry trends, consumer behaviours, and competitor actions to identify opportunities for growth. By leveraging data analytics, businesses can anticipate market shifts and make informed decisions that align with evolving customer needs. Understanding these dynamics helps companies adjust their strategies proactively, enhance customer engagement, and strengthen their competitive edge. Building strong relationships with stakeholders and staying agile in response to changes ensures long-term success in any market.
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 2,449.8 Million |
| Market Size by 2027 | US$ 1,366.0 Million |
| Global CAGR (2020 - 2027) | 7.7% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By 治疗类型
|
| Regions and Countries Covered | 亚太地区
|
| Market leaders and key company profiles |
The regional scope of Asia Pacific Cell Therapy refers to the geographical area in which a business operates and competes. Understanding regional nuances, such as local consumer preferences, economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved regions or adapting their offerings to meet regional demands. A clear regional focus allows for more effective resource allocation, targeted marketing, and better positioning against local competitors, ultimately driving growth in those specific areas.
The Asia Pacific Cell Therapy Market is valued at US$ 2,449.8 Million in 2019, it is projected to reach US$ 1,366.0 Million by 2027.
As per our report Asia Pacific Cell Therapy Market, the market size is valued at US$ 2,449.8 Million in 2019, projecting it to reach US$ 1,366.0 Million by 2027. This translates to a CAGR of approximately 7.7% during the forecast period.
The Asia Pacific Cell Therapy Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Cell Therapy Market report:
The Asia Pacific Cell Therapy Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Cell Therapy Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Cell Therapy Market value chain can benefit from the information contained in a comprehensive market report.