亚太地区生物仿制药市场预测至 2028 年 - COVID-19 影响和按疾病适应症(癌症、糖尿病、自身免疫性疾病和其他疾病)、药物类别(粒细胞集落刺激因子、人类生长激素、胰岛素、TNF 阻滞剂和单克隆抗体)进行的区域分析抗体、促红细胞生成素刺激剂等)、给药途径(静脉内、皮下等)和最终用户(医院、专科诊所、家庭护理等)
No. of Pages: 176 | Report Code: BMIRE00028352 | Category: Life Sciences
No. of Pages: 176 | Report Code: BMIRE00028352 | Category: Life Sciences
生物制剂代表了治疗以前无法治愈的疾病的有前途的新疗法,并且在药品市场中变得非常重要。原研生物制品的专利预计将在未来几年到期。原研生物制品的专利到期和其他知识产权将在未来产生推出新生物仿制药的需求。因此,未来几年该行业市场参与者之间的竞争将加剧。因此,重磅生物制剂的专利到期预计将在预测期内为生物仿制药市场创造利润丰厚的机会。
根据仿制药和生物类似药倡议组织 (GaBI) 的数据,2021 年,国家药品监督管理局 (NMPA) 已批准 13 个仿制药,属于单克隆产品类别抗体和肿瘤坏死因子(TNF)抑制剂,用于中国。
2019年2月,中国首个官方生物类似药获得批准。利妥昔单抗生物类似药HLX01由上海复宏汉霖生物制药研发,用于治疗非霍奇金淋巴瘤(NHL)。 2019年中国又批准了3个生物类似药,2020年批准了7个生物类似药,2019年至2020年增长了75%。绿叶制药集团有限公司的贝伐珠单抗生物类似药于2021年5月获得批准,用于治疗非小细胞肺癌、迈博药业的英夫利昔单抗生物类似药于2021年7月获批用于治疗强直性脊柱炎。
因此,未来中国生物类似药将持续激增,已有11个生物类似药处于预注册阶段正在等待 NMPA 批准,大约 100 种生物仿制药正在开发中。因此,中国强大的生物仿制药管道和国家药品监督管理局的最新监管变化将促进中国生物仿制药市场的增长。
亚太地区生物仿制药市场分为疾病适应症、药物类别、给药途径、最终用户和国家。
<根据疾病适应症,生物仿制药市场分为癌症、糖尿病、自身免疫性疾病和其他疾病适应症。到 2022 年,癌症细分市场占据最大的市场份额。生物仿制药市场根据药物类别分为粒细胞集落刺激因子、人类生长激素、胰岛素、TNF 阻滞剂和单克隆抗体、促红细胞生成素刺激剂等。 2022年粒细胞集落刺激因子细分市场占据最大份额。
根据给药途径,生物仿制药市场分为静脉注射、皮下注射和其他。到 2022 年,静脉注射细分市场将占据最大市场份额。
生物仿制药市场根据最终用户细分为医院、专科诊所、家庭护理、和别的。到 2022 年,医院细分市场将占据最大的市场份额。
按国家/地区划分,亚太地区生物仿制药市场分为澳大利亚、中国、印度、日本、南部非洲韩国以及亚太其他地区。 2022 年中国将主导市场。
Amgen Inc; Sanofi SA;百康有限公司;礼来公司;山德士公司;梯瓦制药工业有限公司;辉瑞公司;和雷迪博士实验室有限公司; Celltrion 公司; Samsung Bioepis Co Ltd 是亚太生物仿制药市场的领先公司。
Strategic insights for Asia Pacific Biosimilars involve closely monitoring industry trends, consumer behaviours, and competitor actions to identify opportunities for growth. By leveraging data analytics, businesses can anticipate market shifts and make informed decisions that align with evolving customer needs. Understanding these dynamics helps companies adjust their strategies proactively, enhance customer engagement, and strengthen their competitive edge. Building strong relationships with stakeholders and staying agile in response to changes ensures long-term success in any market.
| Report Attribute | Details |
|---|---|
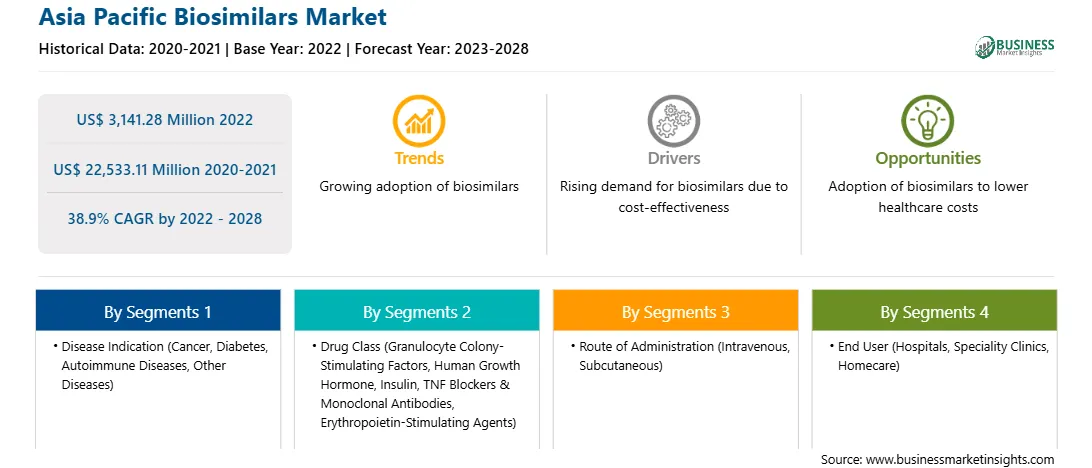
| Market size in 2022 | US$ 3,141.28 Million |
| Market Size by 2028 | US$ 22,533.11 Million |
| Global CAGR (2022 - 2028) | 38.9% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By 疾病适应症
|
| Regions and Countries Covered | 亚太地区
|
| Market leaders and key company profiles |
The regional scope of Asia Pacific Biosimilars refers to the geographical area in which a business operates and competes. Understanding regional nuances, such as local consumer preferences, economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved regions or adapting their offerings to meet regional demands. A clear regional focus allows for more effective resource allocation, targeted marketing, and better positioning against local competitors, ultimately driving growth in those specific areas.
The Asia Pacific Biosimilars Market is valued at US$ 3,141.28 Million in 2022, it is projected to reach US$ 22,533.11 Million by 2028.
As per our report Asia Pacific Biosimilars Market, the market size is valued at US$ 3,141.28 Million in 2022, projecting it to reach US$ 22,533.11 Million by 2028. This translates to a CAGR of approximately 38.9% during the forecast period.
The Asia Pacific Biosimilars Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Biosimilars Market report:
The Asia Pacific Biosimilars Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Biosimilars Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Biosimilars Market value chain can benefit from the information contained in a comprehensive market report.