Prostate cancer is affecting the prostate glands in the male. Prostate cancer is a common cancer following skin cancer in the male. Some common determinants responsible for the start of prostate cancer are family history, old age, and race. Nuclear medicine is imaging that needs radioactive materials. It is a helpful method to identify and also treat prostate cancer and helps radiologists to conclude the stage of cancer. US prostate cancer nuclear medicine diagnostics market is driven by factors such as the rising prevalence of prostate cancer, and the growth of innovative radiopharmaceuticals plays a vital role in the growth of the radiopharmaceuticals market. However, strict guidelines for storage, production, & use of radiopharmaceuticals is likely to obstruct the growth of the US prostate cancer nuclear medicine diagnostics market during the forecast period.
Strategic insights for the US Prostate Cancer Nuclear Medicine Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
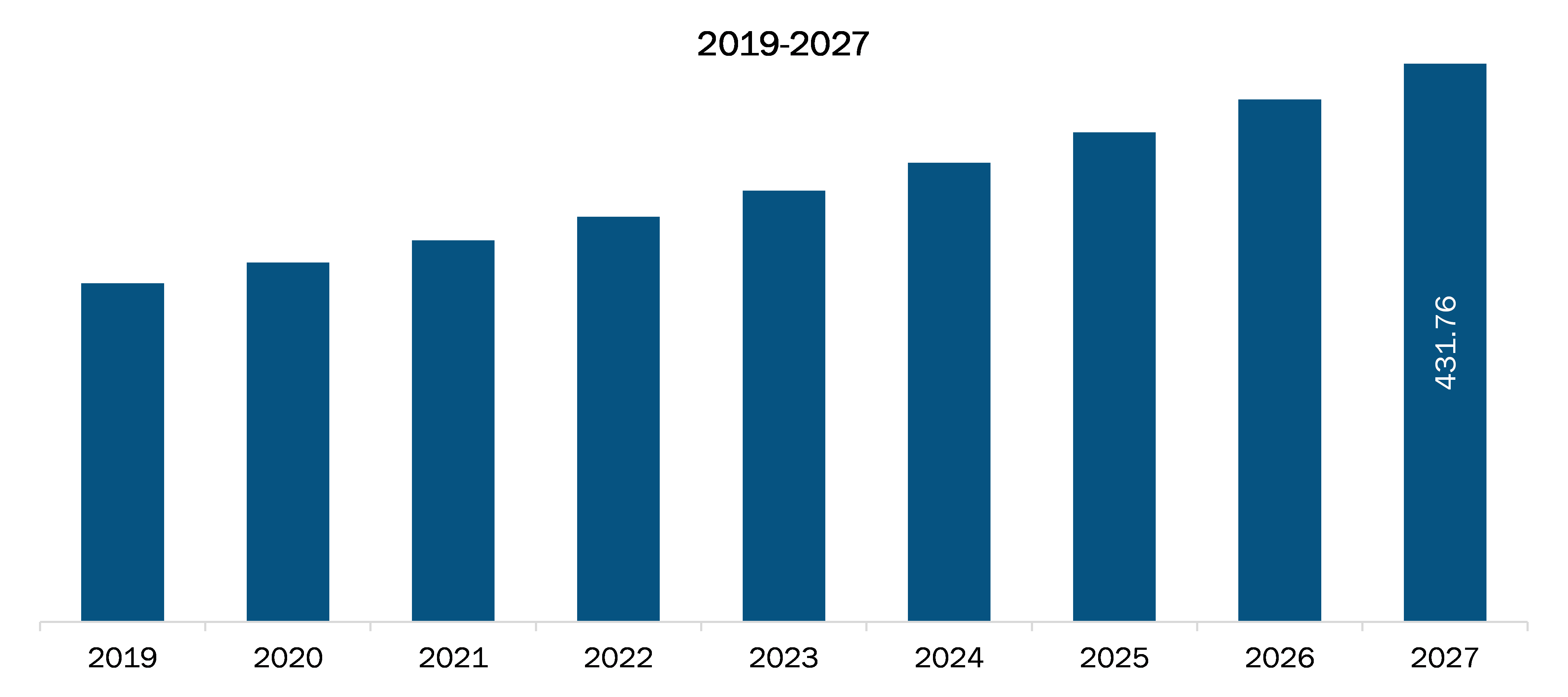
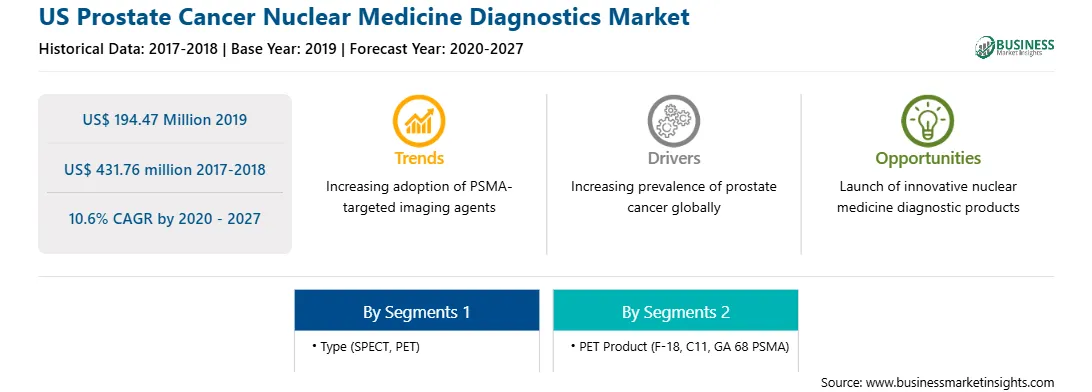
| Market size in 2019 | US$ 194.47 Million |
| Market Size by 2027 | US$ 431.76 million |
| Global CAGR (2020 - 2027) | 10.6% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | United States
|
| Market leaders and key company profiles |
The geographic scope of the US Prostate Cancer Nuclear Medicine Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
Market Insights
Rising Prevalence of Prostate Cancer
Prostate cancer (PCa) is the fourth common cancer across the US in terms of occurrence. The rising prevalence of prostate cancer in the country led to the increased use of nuclear medicines for its treatment. For instance, according to GLOBOCAN, in 2018, there were around 2,129,118 cases of cancer in the US. Among these cases, about 10% were prostate cancer, i.e., 212,783 incidences in 2018. Moreover, the country reported 28,705 deaths due to cancer. The organization has estimated the cases of prostate cancer to reach above 737,463 in the next five years. After skin cancer, prostate cancer is the most common cancer in the American male. As per the data of the American Cancer Society, 2020, it was estimated that prostate cancer would be around 191,930 and approximately 33,330 deaths from prostate cancer in the United States for 2020. Prostate cancer is more likely to grow in older adults. Almost 6 cases in 10 are identified in men who are 65 or more. The average age at identification is around 66 in men in the US. Prostate cancer needs to be examined before the additional treatment begins. And the nuclear medicines are adopted for the diagnosis and analysis ideas. Nuclear medicines in PET and SPECT are accepted for therapeutic and diagnostic prospects. Thus, owing to the rising incidences of prostate cancer poses numerous possibilities for prostate cancer nuclear medicine diagnostics market to increase throughout the forecasted period.
Type Insights
Based on type, the US prostate cancer nuclear medicine diagnostics market is segmented into PET and SPECT. The PET segment held the largest share of the market in 2019 due to higher image quality and sophisticated reimbursement for the procedures as compared to the conventional SPECT nuclear medicine diagnostic processes. Radioisotopes used with PET prostate cancer diagnosis are Fluorine-18, Gallium-68, copper-64, zirconium-89, and Choline-11. Furthermore, the same segment is estimated to register the highest CAGR in the market during the forecast period.
PET Product Insights
The US prostate cancer nuclear medicine diagnostics market, by PET product, is segmented into F-18, C-11, and Ga68-PSMA. The F-18 segment held the largest share of the market in 2019. However, Ga68-PSMA is estimated to register the highest CAGR in the market during the forecast period due to factors such as the rising prevalence of prostate cancer, technological improvements in diagnosis systems using Ga68-PSMA, and its higher sensitivity and accuracy.
Strategic Insights
Product launches and FDA approvals strategy is commonly adopted by companies to expand their footprint worldwide and meet the growing demand. This strategy is most commonly adopted by the market players in order to expand its product portfolio.
The market players operating in the US prostate cancer nuclear medicine diagnostics market adopt the strategy of collaborations to enlarge customer base across the world, which also permits the players to maintain their brand name.
The List of Companies - US Prostate Cancer Nuclear Medicine Market
The US Prostate Cancer Nuclear Medicine Diagnostics Market is valued at US$ 194.47 Million in 2019, it is projected to reach US$ 431.76 million by 2027.
As per our report US Prostate Cancer Nuclear Medicine Diagnostics Market, the market size is valued at US$ 194.47 Million in 2019, projecting it to reach US$ 431.76 million by 2027. This translates to a CAGR of approximately 10.6% during the forecast period.
The US Prostate Cancer Nuclear Medicine Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the US Prostate Cancer Nuclear Medicine Diagnostics Market report:
The US Prostate Cancer Nuclear Medicine Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The US Prostate Cancer Nuclear Medicine Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the US Prostate Cancer Nuclear Medicine Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.