Targeted treatment, faster and more efficient recovery, and reduced side effects are among the advantages of t cell therapy. Globally, cell therapies are widely adopted owing to the availability of various approval:
• In 2023: Kite, a Gilead Company announced launching operations in Saudi Arabia, Singapore, and Brazil as part of its commercial expansion. Kite has filed regulatory applications for its CAR T-cell therapy products in each of the three nations.
• For instance, in June 2022, Bristol Myers Squibb received FDA approval for Breyanzi (lisocabtagene maraleucel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, for the treatment of adult patients with large B-cell lymphoma (LBCL).
• In February 2022, the US Food and Drug Administration (FDA) approved Yescarta (axicabtagene ciloleucel) CAR T-cell therapy for adult patients with large B-cell lymphoma that is refractory to first line chemoimmunotherapy or that relapses within 12 months of first line chemoimmunotherapy. Yescarta is the first CAR T-cell therapy to receive a National Comprehensive Cancer Network (NCCN) Category 1 recommendation.
• In February 2022, the FDA approved ciltacabtagene autoleucel (brand name CARVYKTI) for treating adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
• In January 2022, the FDA approved Kimmtrak (tebentafusp-tebn) for treating unresectable or metastatic uveal melanoma patients who are HLA-A*02:01 positive.
• In March 2021, Abecma (idecabtagene vicleucel) was approved by the FDA for treating relapsed or refractory multiple myeloma. The treatment is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T-cell immunotherapy indicated for treating adult patients with refractory multiple myeloma.
Therefore, the increasing number of approvals for T-cell therapies is fueling the market growth.
The South & Central America T-cell therapy market is limited to Brazil as the products are only approved and commercially available in the country. According to the Brazilian Journal of Cancerology, it is estimated that ~704,000 new cases of cancer are expected during 2023–2025. More than 12,000 non-Hodgkin lymphoma (NHL) cases in Brazil are estimated to be diagnosed yearly. The Brazilian Health Regulatory Agency (ANVISA) has approved two CAR T-cell therapies in Brazil: tisagenlecleucel (by Novartis) for pediatric patients and young adults with RR B-cell acute lymphoblastic leukemia (ALL) and adults with relapsed/refractory (RR) diffuse large B-cell lymphoma, and ciltacabtagene autoleucel (cilta-cel) for patients with relapsed/refractory multiple myeloma (RRMM). Moreover, in August 2022, Kite, a Gilead Company, expanded its business by launching operations in Brazil, Singapore, and Saudi Arabia. Kite has submitted regulatory applications for its CAR T-cell therapy products. The rising cases of cancer and lymphoma, and the increasing number of applications and clinical trials for T-cell therapies are expected to boost the demand in the coming years.
The South & Central America T cell therapy market is segmented into modality, therapy type, indication, and country.
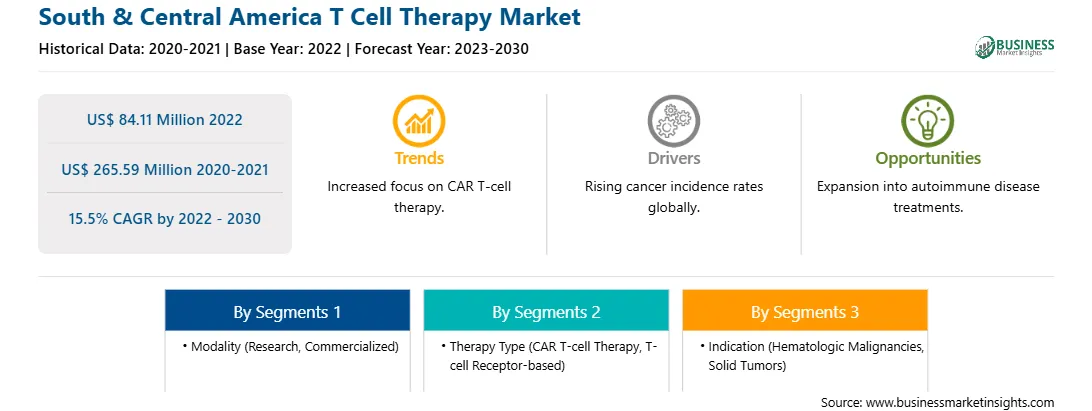
Based on modality, the South & Central America T cell therapy market is bifurcated into research and commercialized. The commercialized segment held a larger market share in 2022.
Based on therapy type, the South & Central America T cell therapy market is divided into CAR T-cell therapy and T-cell Receptor (TCR)-based. The CAR T-cell therapy segment held a larger market share in 2022.
Based on indication, the South & Central America T cell therapy market is bifurcated into hematologic malignancies and solid tumors. The hematologic malignancies segment held the largest market share in 2022.
Based on country, the South & Central America T cell therapy market is limited to Brazil. Brazil dominated the South & Central America T cell therapy market share in 2022.
Bristol-Myers Squibb Co, Gilead Sciences Inc, Janssen Global Services LLC, and Novartis AG are some of the leading companies operating in the South & Central America T cell therapy market.
Strategic insights for the South & Central America T Cell Therapy provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 84.11 Million |
| Market Size by 2030 | US$ 265.59 Million |
| Global CAGR (2022 - 2030) | 15.5% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Modality
|
| Regions and Countries Covered | South and Central America
|
| Market leaders and key company profiles |
The geographic scope of the South & Central America T Cell Therapy refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
1. Bristol-Myers Squibb Co
2. Gilead Sciences Inc
3. Janssen Global Services LLC
4. Novartis AG
The South & Central America T Cell Therapy Market is valued at US$ 84.11 Million in 2022, it is projected to reach US$ 265.59 Million by 2030.
As per our report South & Central America T Cell Therapy Market, the market size is valued at US$ 84.11 Million in 2022, projecting it to reach US$ 265.59 Million by 2030. This translates to a CAGR of approximately 15.5% during the forecast period.
The South & Central America T Cell Therapy Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the South & Central America T Cell Therapy Market report:
The South & Central America T Cell Therapy Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The South & Central America T Cell Therapy Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the South & Central America T Cell Therapy Market value chain can benefit from the information contained in a comprehensive market report.