Molecular quality controls help to monitor the performance of testing procedures. These tests are helpful to achieve critical and accurate diagnostic information. These controls are used to determine transmissible and genetic diseases too. Moreover, the molecular quality controls also help to monitor the performance of nucleic acid testing protocols for healthcare associated infections, viral load assays and sexually transmitted infections.
Strategic insights for the South and Central America Molecular Quality Controls provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.

| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 11.94 Million |
| Market Size by 2028 | US$ 22.84 Million |
| Global CAGR (2021 - 2028) | 9.7% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | South and Central America
|
| Market leaders and key company profiles |
The geographic scope of the South and Central America Molecular Quality Controls refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
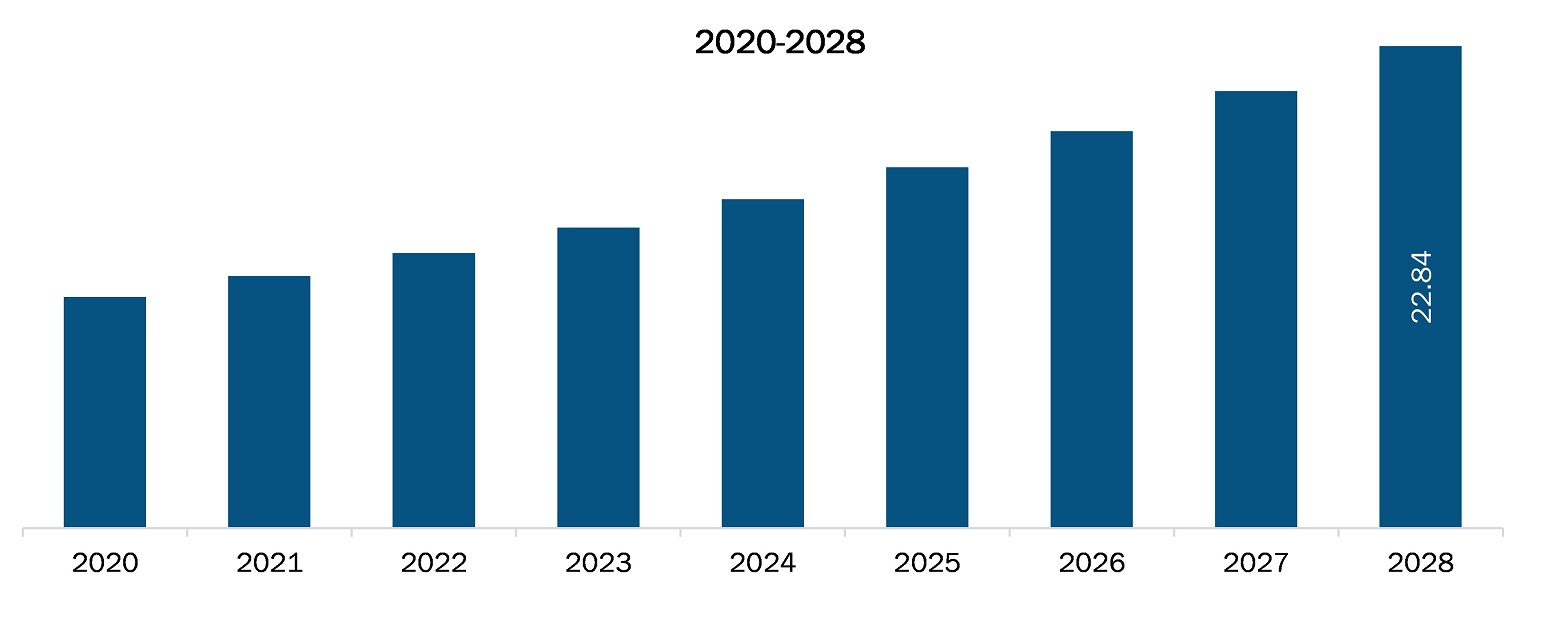
The South and Central America molecular quality controls market is expected to reach US$ 22.84 million in 2028 from US$ 11.94 million in 2021; it is estimated to grow at a CAGR of 9.7% from 2021 to 2028. Factors such as growing funds for genomics, declining cost of sequencing procedures, and increasing prevalence of genetic diseases fuel the growth of the market. However, the dearth of skilled professionals hinders the market growth.
According to the WHO suggestions, 30–50% of deaths caused due to cancer can be prevented by adequate treatment in early stages. In this scenario, personalized medicine offers the most promising approach to tackle diseases that have not been well-known for responding effectively to the existing treatments or cures. Further, digitization is enabling doctors to make decisions about a patient’s cancer treatment, based on the genetic make-up of tumors or cancerous tissues. Thus, these advancements are making personalized medicine a reality by enabling the development of highly targeted therapies that offer the potential for improved treatment outcomes, especially for cancer patients. Moreover, personalized medicine considers genetic, environmental, and lifestyle variability. Hence, the increasing adoption of personalized medicines, based on patient’s genetic information, for disease treatments is expected to emerge as a notable trend in the molecular quality controls market in the coming years. Also, the cost of genome sequencing, as well as next-generation sequencing, has dropped radically in the past 6–7 years. Moreover, the process requires a lesser number of days for completion. Lowering costs and accelerating overall process enable service providers to secure larger profit margins, along with helping them expand their clientele. Thus, the lowered cost of genome sequencing is propelling the number of molecular biology procedures carried out for different purpose, thereby driving the use of molecular quality controls.
South and Central America have been witnessing a growing number of COVID-19 cases since its outbreak. The increasing incidence of COVID-19 supports the growth of the market. International market players are entering the region and offering diagnostic kits for COVID-19. For instance, South Korea’s molecular diagnostics company Seegene Inc. is entering the Brazilian market and launched multi-assay product Allplex, a SARS-CoV-2/FluA/FluB/RSV test which is able to screen and differentiate eight targets. Also, the pharmaceutical & biotechnology industries have invested in R&D for the new molecule entity for quality controls in healthcare. These factors had a positive impact on the South and Central America molecular quality controls market.
The South and Central America molecular quality controls market, by product, is segmented into independent controls and instrument specific controls. The independent controls segment held a larger share of the market in 2020 whereas the same is anticipated to register the highest CAGR in the market during the forecast period.
Based on analyte type, the South and Central America molecular quality controls market has been segmented into single analyte controls and multi-analyte controls. The single analyte controls segment held a larger share of the market in 2020, whereas the multi-analyte control segment is estimated to register the highest CAGR in the market during the forecast period.
The South and Central America molecular quality control market, by application, is segmented into, infectious diseases, oncology, genetic testing, and other applications. The infectious diseases segment held the largest share of the market in 2020, whereas the oncology segment is anticipated to register the highest CAGR in the market during the forecast period.
Based on end user, the South and Central America molecular quality control market is segmented into clinical laboratories, hospitals, IVD manufacturers & contract research organizations, academic & research institutes, and other end users. The clinical laboratories segment held the largest share of the market in 2020 and is estimated to register the highest CAGR in the market during the forecast period.
A few of the primary and secondary sources associated with this report on the South and Central America molecular quality control market are the Biotechnology Industry Research Assistance Council (BIRAC), Council of Scientific & Industrial Research (CSIR) and Center for Cancer Genomics and Advanced Therapeutics(C-CAT).
The South and Central America Molecular Quality Controls Market is valued at US$ 11.94 Million in 2021, it is projected to reach US$ 22.84 Million by 2028.
As per our report South and Central America Molecular Quality Controls Market, the market size is valued at US$ 11.94 Million in 2021, projecting it to reach US$ 22.84 Million by 2028. This translates to a CAGR of approximately 9.7% during the forecast period.
The South and Central America Molecular Quality Controls Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the South and Central America Molecular Quality Controls Market report:
The South and Central America Molecular Quality Controls Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The South and Central America Molecular Quality Controls Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the South and Central America Molecular Quality Controls Market value chain can benefit from the information contained in a comprehensive market report.