South & Central America Electronic Patient-Reported Outcomes (ePROS) Market
No. of Pages: 80 | Report Code: BMIRE00031355 | Category: Technology, Media and Telecommunications
No. of Pages: 80 | Report Code: BMIRE00031355 | Category: Technology, Media and Telecommunications
The South & Central America electronic patient-reported outcomes (ePROS) market was valued at US$ 32.06 million in 2023 and is expected to reach US$ 86.04 million by 2031; it is estimated to register a CAGR of 13.1% from 2023 to 2031.
The market for electronically reported patient outcomes (ePROs) is expected to experience lucrative opportunities with the rising adoption of telehealth and remote monitoring for clinical trials during the forecast period. Patients in remote or underserved areas can report real-time information from the comfort of their homes owing to telehealth and remote monitoring solutions, which increase accessibility of patient data. Health data can be continuously transmitted via remote monitoring devices, such as wearables and linked health systems. When coupled with ePRO systems, medical professionals receive detailed information regarding a patient's condition in real time, enabling more precise and regular interventions. For example, Vivalink's integrated acute remote patient monitoring solution provides continuous and real-time monitoring of patient vitals, including live ECG, as well as information about vitals and biometrics, EPRO/ECOA/surveys, centralized data services, remote data collection, and patient adherence. The efficacy of ePRO systems is expanded by telehealth and remote monitoring developments, establishing them as a crucial element of patient-centered care.
Brazil is a significant destination for clinical trials. According to Clinical Trials Arena, Brazil accounted for 1.7% share of the overall clinical trials activities worldwide in 2021. Boston CRO acquired Rio de Janeiro-based Instituto Brasil de Pesquisa Clinica (IBPClin) in July 2022, describing it as the first step toward adopting its decentralized clinical trial delivery model in Latin America. IBPClin claims to have conducted over 160 industry-sponsored research studies, registering more than 7,000 participants across 12 Brazilian states. In addition, digital initiatives for the transformation of healthcare in the country are rapidly increasing. For instance, The SUS Digital is driving the digital transformation of the healthcare system in Brazil. The initiative expands citizens' access to healthcare services by incorporating technologies, thereby promoting a more integrated and efficient approach to healthcare. The program also covers data monitoring and evaluation, information systems, platforms, and application development. Therefore, the increasing number of clinical trials and surging digital transformation of the healthcare system in the country are likely to contribute the market growth in the coming years.
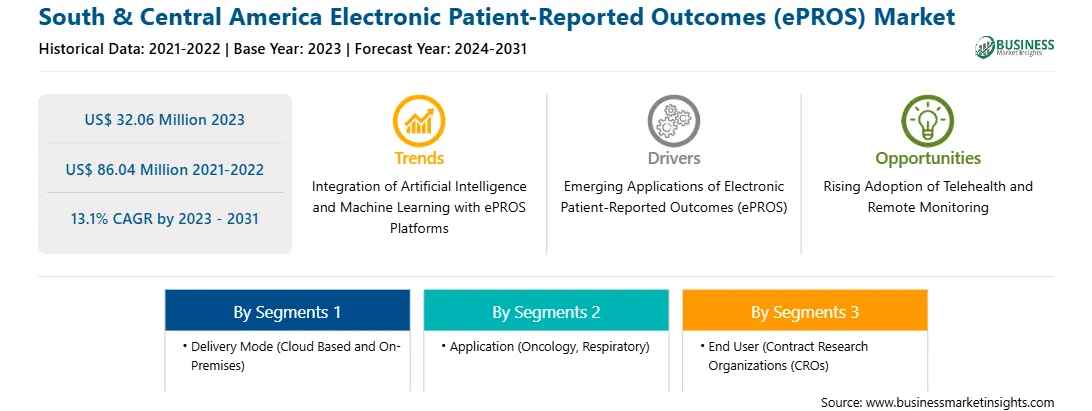
Strategic insights for the South & Central America Electronic Patient-Reported Outcomes (ePROS) provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the South & Central America Electronic Patient-Reported Outcomes (ePROS) refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.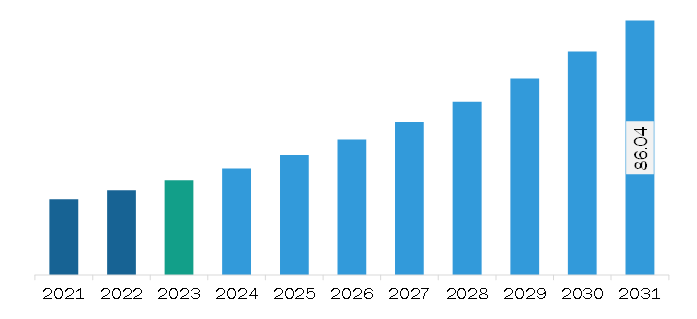
South & Central America Electronic Patient-Reported Outcomes (ePROS) Strategic Insights
South & Central America Electronic Patient-Reported Outcomes (ePROS) Report Scope
Report Attribute
Details
Market size in 2023
US$ 32.06 Million
Market Size by 2031
US$ 86.04 Million
Global CAGR (2023 - 2031)
13.1%
Historical Data
2021-2022
Forecast period
2024-2031
Segments Covered
By Delivery Mode
By Application
By End User
Regions and Countries Covered
South and Central America
Market leaders and key company profiles
South & Central America Electronic Patient-Reported Outcomes (ePROS) Regional Insights
The South & Central America electronic patient-reported outcomes (ePROS) market is categorized into delivery mode, application, end user, and country.
By delivery mode, the South & Central America electronic patient-reported outcomes (ePROS) market is bifurcated into cloud based and on-premises. The cloud based segment held a larger share of the South & Central America electronic patient-reported outcomes (ePROS) market share in 2023.
In terms of application, the South & Central America electronic patient-reported outcomes (ePROS) market is segmented into oncology, respiratory, and others. The oncology segment held the largest share of the South & Central America electronic patient-reported outcomes (ePROS) market share in 2023.
By end user, the South & Central America electronic patient-reported outcomes (ePROS) market is segmented into contract research organizations (CROs), pharmaceutical companies, and others. The pharmaceutical companies segment held the largest share of the South & Central America electronic patient-reported outcomes (ePROS) market share in 2023.
Based on country, the South & Central America electronic patient-reported outcomes (ePROS) market is segmented into Brazil, Argentina, and the Rest of South & Central America. Brazil segment held the largest share of South & Central America electronic patient-reported outcomes (ePROS) market in 2023.
Assistek, Buddy Healthcare Ltd Oy, Castor, Clinical Ink Inc, Crucial Data Solutions, Curebase, Medable Inc, Medidata Solutions, Medrio, OpenClinica LLC, PatientIQ, Signant Health, Veeva Systems Inc, and Y-Prime LLC are some of the leading companies operating in the South & Central America electronic patient-reported outcomes (ePROS) market.
The South & Central America Electronic Patient-Reported Outcomes (ePROS) Market is valued at US$ 32.06 Million in 2023, it is projected to reach US$ 86.04 Million by 2031.
As per our report South & Central America Electronic Patient-Reported Outcomes (ePROS) Market, the market size is valued at US$ 32.06 Million in 2023, projecting it to reach US$ 86.04 Million by 2031. This translates to a CAGR of approximately 13.1% during the forecast period.
The South & Central America Electronic Patient-Reported Outcomes (ePROS) Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the South & Central America Electronic Patient-Reported Outcomes (ePROS) Market report:
The South & Central America Electronic Patient-Reported Outcomes (ePROS) Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The South & Central America Electronic Patient-Reported Outcomes (ePROS) Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the South & Central America Electronic Patient-Reported Outcomes (ePROS) Market value chain can benefit from the information contained in a comprehensive market report.