In decentralized clinical trials (DCT), patients' physical access to hospital-based trial sites is reduced or eliminated. In DCTs, digital technologies are used to enable access of patients to clinical research, remote data collection and monitoring, and communication between investigators and participants. A hybrid clinical trial approach combines home-based and on-site activities, bringing the best patient experience and meeting complex protocol regimes, gaining traction across various therapeutic areas and trial phase journeys. Initially, due to challenges such as patient privacy, data security, regulatory barriers, and complex protocol regimes, the adoption of DCT was affected. However, due to the COVID-19 pandemic, the sponsors of clinical trials adopted decentralized and hybrid clinical techniques for developing drugs. They could not continue with traditional trials as they required in-person visits. Due to the restrictions for travel, the only way to gather data and keep trials going was to work remotely and adopt technology much faster than they would have otherwise. Therefore, decentralization broadens trial access to reach a larger number and potentially a more diverse pool of patients.
Further, hybrid clinical trials allow sponsors to strategically incorporate decentralized clinical trial (DCT) elements into study designs. These trial models offer unprecedented flexibility, so more companies are interested in hybrid trials, and the resulting growth is redefining the industry landscape. According to ObvioHealth, the FDA plans to unveil protocols in 2023 to support the use of DCT methods, inspiring confidence in these components for future clinical research. Thus, the increasing focus on using decentralized and hybrid clinical trials over traditional clinical trial methods is expected to provide lucrative opportunities for the clinical trials market during the forecast period.
The South & Central America clinical trials market is segmented into Brazil, Argentina, and the Rest of South & Central America. South & Central America occupies a significant position in the clinical trials market and is estimated to register a robust growth rate over the forecast period. The growth is due to growing clinical trials, key innovation by market players of clinical trial process, and government initiatives to support the clinical trial fuels the market growth.
Strategic insights for the South & Central America Clinical Trials provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the South & Central America Clinical Trials refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.South & Central America Clinical Trials Strategic Insights
South & Central America Clinical Trials Report Scope
Report Attribute
Details
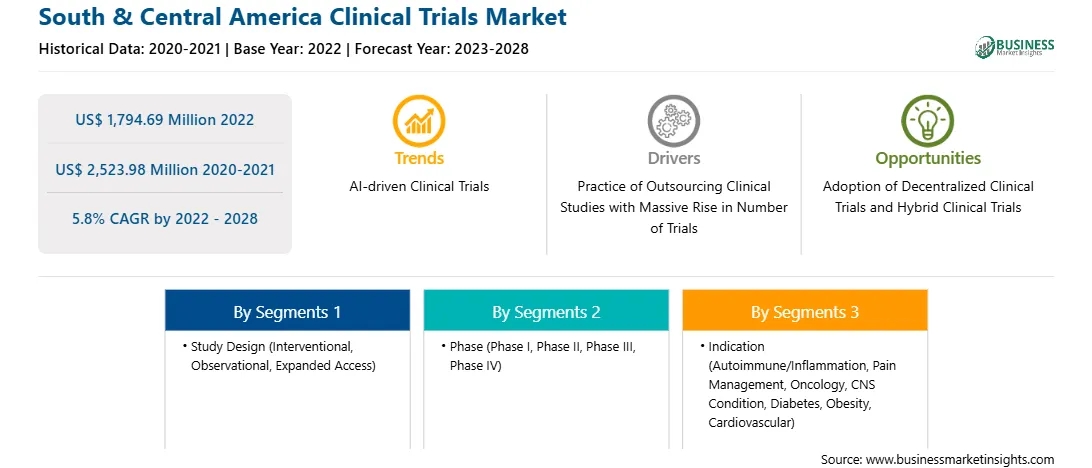
Market size in 2022
US$ 1,794.69 Million
Market Size by 2028
US$ 2,523.98 Million
Global CAGR (2022 - 2028)
5.8%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Study Design
By Phase
By Indication
Regions and Countries Covered
South and Central America
Market leaders and key company profiles
South & Central America Clinical Trials Regional Insights
South & Central America Clinical Trials Market Segmentation
The South & Central America clinical trials market is segmented into phase, study design, indication, and country.
Based on phase, the South & Central America clinical trials market is segmented into phase I, phase II, phase III, and phase IV. The phase III segment registered the largest South & Central America clinical trials market share in 2022.
Based on study design, the South & Central America clinical trials market is segmented into interventional, observational, and expanded access. The interventional segment held the largest South & Central America clinical trials market share in 2022.
Based on indication, the South & Central America clinical trials market is segmented into autoimmune/inflammation, pain management, oncology, CNS condition, diabetes, obesity, cardiovascular, and others. The oncology segment held the largest South & Central America clinical trials market share in 2022.
Based on country, the South & Central America clinical trials market has been categorized into Brazil, Argentina, and the Rest of South & Central America. The Rest of South & Central America dominated the South & Central America clinical trials market share in 2022.
Charles River Laboratories International Inc, ICON Plc, IQVIA Holdings Inc, Laboratory Corp of America Holdings, Parexel International Corp, SGS SA, Syneos Health Inc, and Thermo Fisher Scientific Inc. are some of the leading companies operating in the South & Central America clinical trials market.
The South & Central America Clinical Trials Market is valued at US$ 1,794.69 Million in 2022, it is projected to reach US$ 2,523.98 Million by 2028.
As per our report South & Central America Clinical Trials Market, the market size is valued at US$ 1,794.69 Million in 2022, projecting it to reach US$ 2,523.98 Million by 2028. This translates to a CAGR of approximately 5.8% during the forecast period.
The South & Central America Clinical Trials Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the South & Central America Clinical Trials Market report:
The South & Central America Clinical Trials Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The South & Central America Clinical Trials Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the South & Central America Clinical Trials Market value chain can benefit from the information contained in a comprehensive market report.