South & Central America Cell and Gene Therapy Manufacturing Services Market
No. of Pages: 92 | Report Code: BMIRE00029316 | Category: Life Sciences
No. of Pages: 92 | Report Code: BMIRE00029316 | Category: Life Sciences
Cell and gene therapy manufacturing is a complex process, which makes the proper execution and overseeing of the operation crucial. Cell and gene therapy manufacturers have a limited number of qualified personnel who know biological and process engineering. Moreover, for experienced teams, managing the attempts to reach the first clinical trial using a manual, and open manufacturing method and then building a more commercially suitable process can be tricky. Therefore, these enterprises choose to work with contract development and manufacturing organizations (CDMOs) to accelerate their clinical studies and commercialization process. CDMOs provide product development, manufacturing, clinical trial support, and commercialization services to cell and gene therapy companies on a contract basis. Partnering with a CDMO enables scalability, speed to market, access to technical expertise without overhead costs, and cost efficiencies for cell and gene therapy manufacturers. Further, in March 2020, Fujifilm Cellular Dynamics (FCDI) invested US$ 21 million in the cGMP-compliant production facility, which would be used for manufacturing FCDI’s pipeline of regenerative medicine therapies using induced pluripotent stem cells (iPSCs) and to provide CDMO services for production of iPSCs and iPSC-derived differentiated cells. Outsourcing cell and gene therapy manufacturing to CDMOs proves cost-effective for manufacturers. Moreover, they gain access to the technologically advanced infrastructure and expertise of CDMOs. CDMOs employ proper, mapped processes for manufacturing cell and gene therapies. Thus, the increasing preference for outsourcing growing cell and gene therapy manufacturing to CDMOs fuels the South & Central America cell and gene therapy manufacturing services market growth.
According to a report titled "First Gene Therapy Products Approved in Brazil," published by Law Business Research 2020, Brazil is the first among the Latin American countries to approve the marketing of gene therapy products. Specifically, the Brazilian Health Regulatory Agency (ANVISA) granted marketing authorization for Novartis’ gene therapy products Luxturna and Zolgensma. Both products have been approved by the Brazilian Technical Commission of Biosafety (CTNBio), which is responsible for evaluating the biosafety of genetically modified organisms in Brazil. According to Clinical Trials Arena, in 2021, Brazil accounted for a 1.7% share of the global clinical trials activity. In July 2022, Boston CRO acquired Rio de Janeiro-based Instituto Brasil de Pesquisa Clinica (IBPClin), describing it as the first step toward adopting its decentralized clinical trial delivery model in Latin America. It claims to have conducted more than 160 industry-sponsored research studies, enrolling more than 7,000 participants across 12 Brazilian states.
The South & Central America cell and gene therapy manufacturing services market is segmented into type, indication, application, end user, and country.
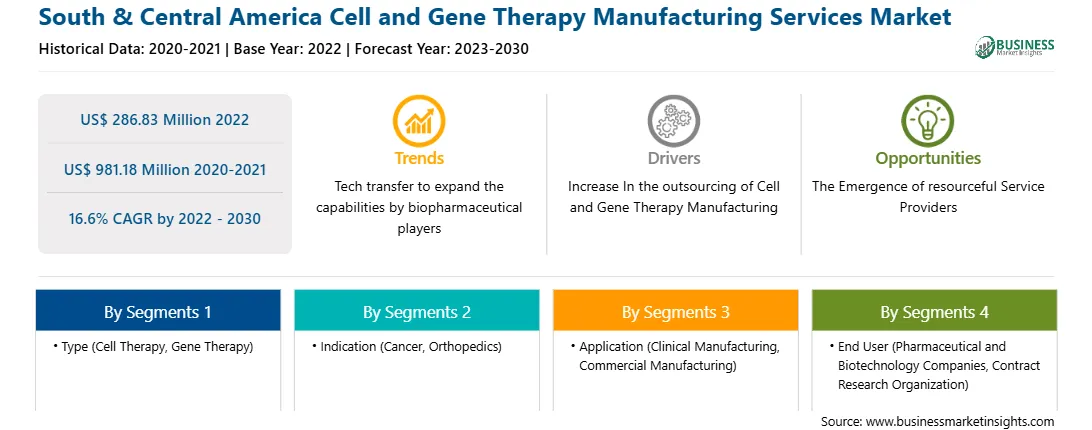
Based on type, the South & Central America cell and gene therapy manufacturing services market is bifurcated into cell therapy and gene therapy. In 2022, the cell therapy segment registered a larger share in the South & Central America cell and gene therapy manufacturing services market. The cell therapy segment is further segmented into autologous and allogenic. The gene therapy segment is further segmented into viral and non-viral vector.
Based on indication, the South & Central America cell and gene therapy manufacturing services market is segmented into cancer, orthopedics, and others. In 2022, the cancer segment registered the largest share in the South & Central America cell and gene therapy manufacturing services market.
Based on application, the South & Central America cell and gene therapy manufacturing services market is segmented into clinical manufacturing and commercial manufacturing. In 2022, the commercial manufacturing segment registered the largest share in the South & Central America cell and gene therapy manufacturing services market.
Based on end user, the South & Central America cell and gene therapy manufacturing services market is bifurcated into pharmaceutical and biotechnology companies and contract research organization (CROs). In 2022, the pharmaceutical and biotechnology companies segment registered a larger share in the South & Central America cell and gene therapy manufacturing services market.
Based on country, the South & Central America cell and gene therapy manufacturing services market is segmented into Brazil, Argentina, and the Rest of South & Central America. In 2022, Brazil registered the largest share in the South & Central America cell and gene therapy manufacturing services market.
Catalent Inc, Charles River Laboratories International Inc, Lonza Group AG, Merck KgaA, Nikon Corp, Takara Bio Inc, and Thermo Fisher Scientific Inc are some of the leading companies operating in the South & Central America cell and gene therapy manufacturing services market.
Strategic insights for the South & Central America Cell and Gene Therapy Manufacturing Services provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 286.83 Million |
| Market Size by 2030 | US$ 981.18 Million |
| Global CAGR (2022 - 2030) | 16.6% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | South and Central America
|
| Market leaders and key company profiles |
The geographic scope of the South & Central America Cell and Gene Therapy Manufacturing Services refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
1. Catalent Inc
2. Charles River Laboratories International Inc
3. Lonza Group AG
4. Merck KgaA
5. Nikon Corp
6. Takara Bio Inc
7. Thermo Fisher Scientific Inc
The South & Central America Cell and Gene Therapy Manufacturing Services Market is valued at US$ 286.83 Million in 2022, it is projected to reach US$ 981.18 Million by 2030.
As per our report South & Central America Cell and Gene Therapy Manufacturing Services Market, the market size is valued at US$ 286.83 Million in 2022, projecting it to reach US$ 981.18 Million by 2030. This translates to a CAGR of approximately 16.6% during the forecast period.
The South & Central America Cell and Gene Therapy Manufacturing Services Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the South & Central America Cell and Gene Therapy Manufacturing Services Market report:
The South & Central America Cell and Gene Therapy Manufacturing Services Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The South & Central America Cell and Gene Therapy Manufacturing Services Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the South & Central America Cell and Gene Therapy Manufacturing Services Market value chain can benefit from the information contained in a comprehensive market report.