Market Introduction
South America region for the estimating the market for the Healthcare Regulatory Affairs Outsourcing consist of countries such as Brazil, Argentina, and rest of South America. The region held smallest portion of the market in the global analysis. The market is likely to propel in the forecasted period owing to the cost savings advantage and improve the efficiency of clinical trials. Moreover, developing healthcare infrastructure, growing public-private investments for clinical research, and growing awareness about the benefits of regulatory affairs outsourcing among the healthcare industry. Brazil is the fifth largest country globally in the world by area and by population and experiencing the fastest technological advancements and which is expected to serve wider growth opportunities for market growth. In Brazil, the biotechnology and pharmaceutical market has experienced several activities in terms of industrial-scale activities and healthcare. Brazil is known for its research and investment in industrial biotechnology, predominantly regarding clinical trial services. The Brazilian stakeholders, policymakers, and public investors have promoted the advancement of healthcare research in Brazil. The trade groups in Brazil aim to enhance the current patent laws to support developments, healthcare infrastructures, and knowledge transfer by increasing the training activities. These factors are expected to offer several opportunities for healthcare regulatory affairs outsourcing market during the forecast years. Accelerating regulatory pressure on healthcare companies is the major factor driving the growth of the SAM healthcare regulatory affairs outsourcing market.
South American countries have been witnessing a growing number of COVID-19 cases since the disease outbreak in the world. According to Worldometer, the number of cases reached 21,781,436 in Brazil and 5,286,074 in Argentina as of October 2021. The pandemic is expected to push the region its worst recession so far, causing a 9.1% contraction in regional GDP in 2020. It is presenting critical challenges for the already overburdened and underfunded public healthcare systems of South America. Governments of these countries have responded to the situation by closing borders, ordering quarantines, and imposing a host of restrictions to keep people confined at home. Currently, the governments are rapidly increasing their clinical programs to fight against the novel coronavirus. Many manufacturing plants have been shut down amid the pandemic. The majority of the manufacturing companies in the region are concentrated on developing therapeutics for the disease. While regulatory outsourcing methods in clinical trials are not new to this region, the pandemic has accelerated their adoption. As physicians and patients have become more receptive of digital technologies and teleconferencing, more opportunities for the proliferation of the remote monitoring segment are being generated. Drug developers are challenged to rapidly assess the effectiveness of existing vaccines against evolving SARS-CoV-2 strains due to the virus's mutations. As a result, increased visibility and oversight of data collection, faster trial implementation, and real-time data sharing have become top priorities of the pharmaceutical companies. These factors have a positive impact on the potential South America healthcare regulatory affairs outsourcing market growth.
Strategic insights for the South America Healthcare Regulatory Affairs Outsourcing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the South America Healthcare Regulatory Affairs Outsourcing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
South America Healthcare Regulatory Affairs Outsourcing Strategic Insights
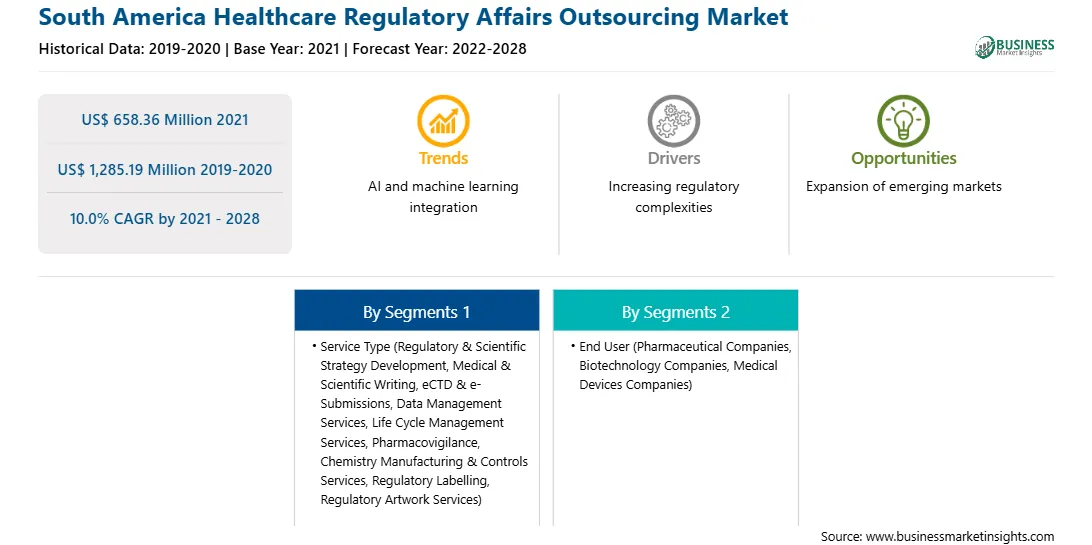
South America Healthcare Regulatory Affairs Outsourcing Report Scope
Report Attribute
Details
Market size in 2021
US$ 658.36 Million
Market Size by 2028
US$ 1,285.19 Million
Global CAGR (2021 - 2028)
10.0%
Historical Data
2019-2020
Forecast period
2022-2028
Segments Covered
By Service Type
By End User
Regions and Countries Covered
South and Central America
Market leaders and key company profiles
South America Healthcare Regulatory Affairs Outsourcing Regional Insights
Market Overview and Dynamics
The healthcare regulatory affairs outsourcing market in SAM is expected to grow from US$ 658.36 million in 2021 to US$ 1,285.19 million by 2028; it is estimated to grow at a CAGR of 10.0% from 2021 to 2028. The development of blockbuster therapies such as targeted gene therapies, specialty drugs, and precision medicine that help treat specific diseases and disorders has been a major focus in the healthcare sector for a long period. A few of these therapies are also being combined with medical devices to enhance the quality of drug delivery, dose, and patient monitoring or adherence, which is expected to add to the complexity of the related regulatory strategies and difficulties in their way to market. Thus, developments in emerging segments in healthcare sectors such as specialty therapies, orphan drugs, and personalized medicines are expected to offer significant growth opportunities to healthcare regulatory affairs outsourcing market players during the forecast period.
Key Market Segments
The SAM healthcare regulatory affairs outsourcing market has been segmented based on service type, end user, and country. On the basis of service type, the SAM healthcare regulatory affairs outsourcing market is segmented into medical & scientific writing, pharmacovigilance, data management services, life cycle management services, eCTD and e-Submissions, regulatory and scientific strategy development, chemistry manufacturing and controls (CMC) services, regulatory labelling, and regulatory artwork services. The medical & scientific writing segment dominated the market in 2020 and pharmacovigilance segment is expected to be the fastest growing during the forecast period. Based on end user, the market is segmented into pharmaceutical companies, biotechnology companies, and medical devices companies. The pharmaceutical companies segment dominated the market in 2020 and is expected to be the fastest growing during the forecast period. Likewise, the medical devices companies segmented is categorized into medical device materials & biomaterials, medical device, biomarkers and in vitro diagnostics (IVD), medical device software (SaMD), medical device electromechanics, medical device substance-based, and medical device of combination product.
Major Sources and Companies Listed
A few major primary and secondary sources referred to for preparing this report on healthcare regulatory affairs outsourcing market in SAM are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Arriello Ireland Ltd., Azierta Contract Science Support Consulting, IQVIA Inc., PAREXEL INTERNATIONAL CORPORATION, PHARMALEX GMBH, and ProductLife Group are among others.
Reasons to buy report
SAM Healthcare Regulatory Affairs Outsourcing Market Segmentation
SAM Healthcare Regulatory Affairs Outsourcing Market –By
Service Type
SAM Healthcare Regulatory Affairs Outsourcing Market –By End User
SAM Healthcare Regulatory Affairs Outsourcing Market -By Country
SAM Healthcare Regulatory Affairs Outsourcing Market -
Company Profiles
The South America Healthcare Regulatory Affairs Outsourcing Market is valued at US$ 658.36 Million in 2021, it is projected to reach US$ 1,285.19 Million by 2028.
As per our report South America Healthcare Regulatory Affairs Outsourcing Market, the market size is valued at US$ 658.36 Million in 2021, projecting it to reach US$ 1,285.19 Million by 2028. This translates to a CAGR of approximately 10.0% during the forecast period.
The South America Healthcare Regulatory Affairs Outsourcing Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the South America Healthcare Regulatory Affairs Outsourcing Market report:
The South America Healthcare Regulatory Affairs Outsourcing Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The South America Healthcare Regulatory Affairs Outsourcing Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the South America Healthcare Regulatory Affairs Outsourcing Market value chain can benefit from the information contained in a comprehensive market report.