The North America vaccine adjuvants market was valued at US$ 1,044.84 million in 2022 and is expected to reach US$ 2,862.04 million by 2030; it is estimated to register a CAGR of 13.4% from 2022 to 2030.
According to the World Bank 2023 report, bacterial, viral, parasite, or fungal diseases can be contagious, spreading directly from person to person or animal to person. As per the National Institute of Health (NIH) report, the H1N1 influenza pandemic resulted in losses of ~US$ 2.8 billion to the Mexican tourism industry, as the footfall of tourists decreased by nearly 1 million over a 5-month window of the contagious spread of H1N1 virus. The Mexican state of Texcoco reported an outbreak of low pathogenicity avian influenza (LPAI) A(H5N2) in March 2024, and Temascalapa municipality in the same state reported another outbreak in April. Additionally, in the state of Michoacán, a private poultry farm detected a high-pathogenicity avian influenza A(H5N2) outbreak that was discovered in March 2024. In 2022, it was reported that both LPAI and HPAI A(H5) subtypes have been found in bird species in Mexico, both recently and in the past. Although they typically infect animals, animal influenza viruses can also infect people. Human infections have mostly been contracted via close contact with animals that are infected or contaminated environments. Influenza A viruses can be categorized as avian influenza, swine influenza, or other animal influenza viruses based on their original host. Therefore, the emergence of various infectious diseases with the associated cost burden accelerates the manufacturers' demand for vaccines and vaccine adjuvants.
COVAXIN, a vaccine developed by Bharat Biotech to fight against the COVID-19 pandemic, uses Alhydroxiquim-II as an adjuvant. The biotech company ViroVax LLC discovered and tested this adjuvant in the laboratory. The pharmacological action of Alhydroxiquim-II is that it travels through lymph nodes and activates TLR7 and TLR8 cellular receptors, which play a vital role in the immune response against viruses. Alhydroxiquim-II is the first adjuvant responsible for activating TLR7 and TLR8, thus stimulating people’s immune responses. Therefore, the rising number of infectious disease outbreaks and pandemics, accounting for high healthcare costs, propels the demand for vaccine adjuvants.
The North America vaccine adjuvants market has been segmented into the US, Canada, and Mexico. Fast product approval processes benefit the vaccine adjuvants market in the US. In May 2023, GlaxoSmithKline (GSK) won US Food and Drug Administration (FDA) approval for its Arexvy (respiratory syncytial virus vaccine, adjuvanted) vaccine intended for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV). Thus, Arexvy became the world's first FDA-approved RSV vaccine for older adults. In October 2022, Novavax, Inc. received an Emergency Use Authorization (EUA) from the FDA for its COVID-19 adjuvanted vaccine, indicated as a booster for adult patients.
Strategic insights for the North America Vaccine Adjuvants provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the North America Vaccine Adjuvants refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.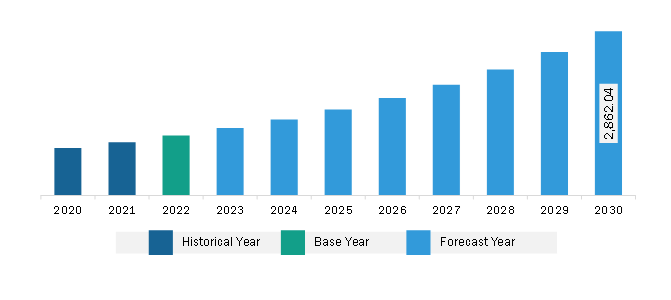
North America Vaccine Adjuvants Strategic Insights

North America Vaccine Adjuvants Report Scope
Report Attribute
Details
Market size in 2022
US$ 1,044.84 Million
Market Size by 2030
US$ 2,862.04 Million
Global CAGR (2022 - 2030)
13.4%
Historical Data
2020-2021
Forecast period
2023-2030
Segments Covered
By Adjuvant Class
By Type
Regions and Countries Covered
North America
Market leaders and key company profiles
North America Vaccine Adjuvants Regional Insights
North America Vaccine Adjuvants Market Segmentation
The North America vaccine adjuvants market is categorized into adjuvant class, type, and country.
Based on adjuvant class, the North America vaccine adjuvants market is segmented into mineral salt adjuvant, emulsion adjuvant, liposome adjuvant, and others. The mineral salt adjuvant segment held the largest share of the North America vaccine adjuvants market share in 2022.
By type, the North America vaccine adjuvants market is bifurcated into human vaccine adjuvant and veterinary vaccine adjuvant. The human vaccine adjuvant segment held a larger share of North America vaccine adjuvants market in 2022.
By country, the North America vaccine adjuvants market is segmented into the US, Canada, and Mexico. The US dominated the North America vaccine adjuvants market share in 2022.
Croda International Plc; CSL Ltd; Dynavax Technologies Corp; GSK Plc; Hawaii Biotech Inc; InvivoGen SAS; Novavax Inc; Phibro Animal Health Corp; Seppic SA; and SPI Pharma Inc are some of the leading companies operating in the North America vaccine adjuvants market.
The North America Vaccine Adjuvants Market is valued at US$ 1,044.84 Million in 2022, it is projected to reach US$ 2,862.04 Million by 2030.
As per our report North America Vaccine Adjuvants Market, the market size is valued at US$ 1,044.84 Million in 2022, projecting it to reach US$ 2,862.04 Million by 2030. This translates to a CAGR of approximately 13.4% during the forecast period.
The North America Vaccine Adjuvants Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Vaccine Adjuvants Market report:
The North America Vaccine Adjuvants Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Vaccine Adjuvants Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Vaccine Adjuvants Market value chain can benefit from the information contained in a comprehensive market report.