North America Software as a Medical Device Market
No. of Pages: 110 | Report Code: TIPRE00010412 | Category: Technology, Media and Telecommunications
No. of Pages: 110 | Report Code: TIPRE00010412 | Category: Technology, Media and Telecommunications
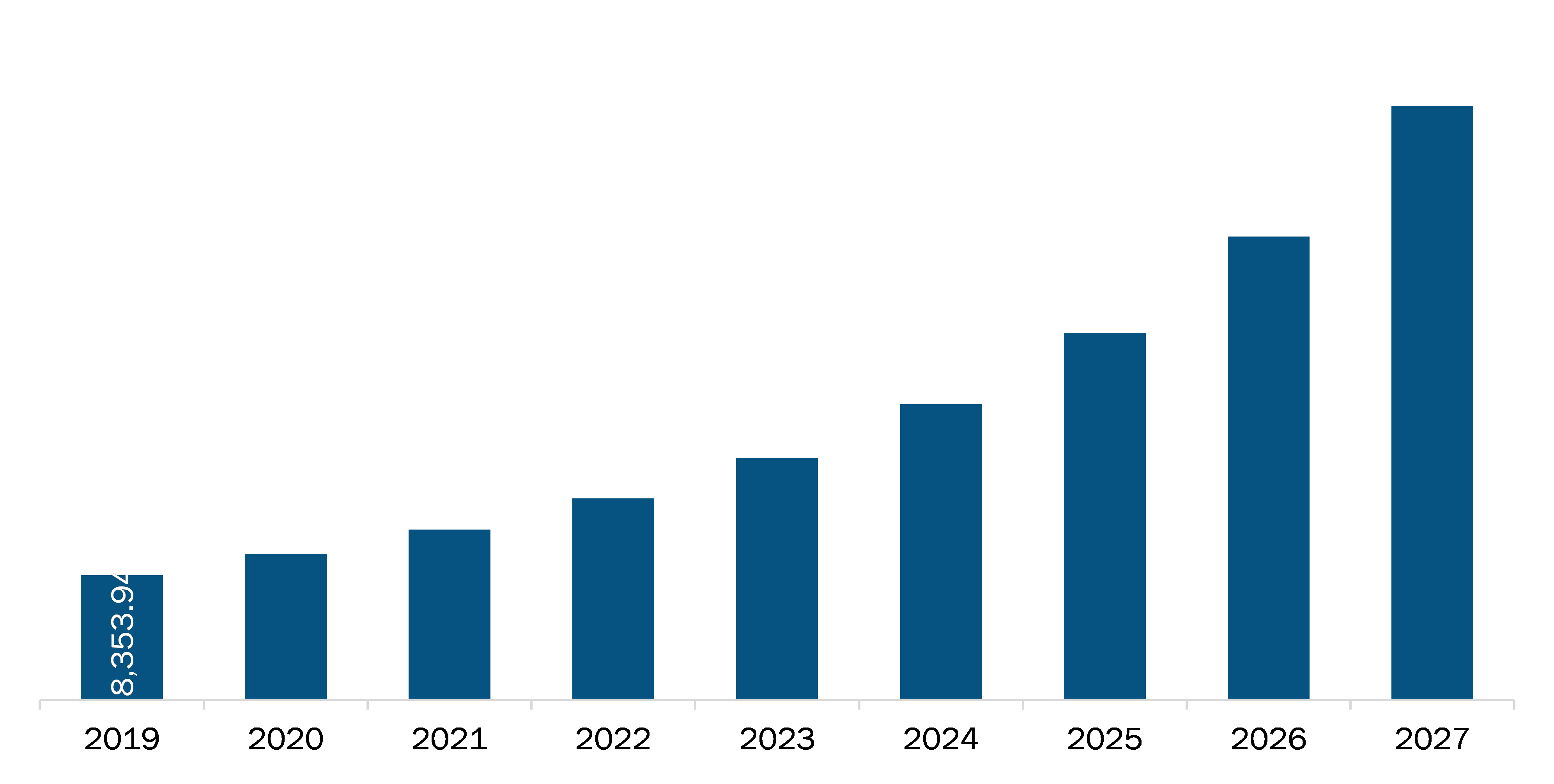
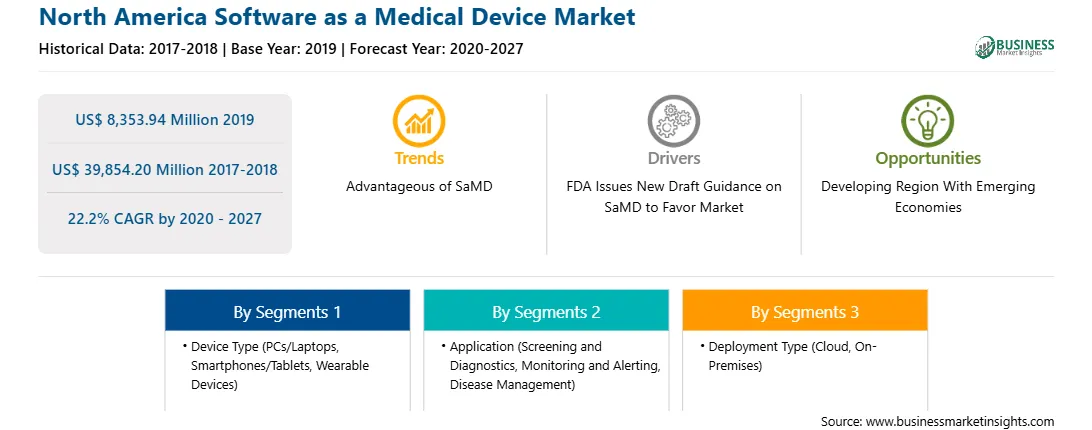
The North America Software as Medical Device market is expected to reach US$ 39,854.20 Mn in 2027 from US$ 8,353.94 Mn in 2019. The market is estimated to grow with a CAGR of 22.2% from 2020-2027.
The growth of the market is driven by the factors such as, increasing adoption of IoT in healthcare and advantages of software as medical device (SaMD) .On the other hand, threat of data breach is likely to restraint the growth of market during the forecast years.
The North American Software as Medical Device market is expected to witness significant growth during the forecast period, due to factors such as the rising adoption of Internet of Things (IoT) in healthcare services for screening and monitoring of patient may offer vital growth opportunities for market growth. The US is among the top countries in the field of software as medical device. The presence of key players in the country and guidelines proposed by the US FDA for software as medical device are driving the growth of software as medical device in country.
The growth of the North American software as a medical device market is primarily driven by increasing adoption of smart health monitoring devices, transformation of digital healthcare, increasing number of chronic diseases, and support from the federal governments to implement digital tools in healthcare to cut down the costs and improve the quality of care. According to the US FDA, software has become an important part of all the products that are widely integrated into the digital healthcare platforms. The FDA also states that the use of software as medical device is continuously increasing in the US. The implementation of these devices is broadly seen across a range of digital platforms, including commercial off-shelf platforms, virtual networks, and medical device platforms. The International Medical Device Regulators Forum (IMDRF) is a voluntary group of medical device regulators that have come together to develop a harmonized regulation on medical devices. The US FDA specifies certain criteria to qualify software as medical devices.
Strategic insights for the North America Software as a Medical Device provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 8,353.94 Million |
| Market Size by 2027 | US$ 39,854.20 Million |
| Global CAGR (2020 - 2027) | 22.2% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Device Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America Software as a Medical Device refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
North America Software as Medical Device – Market Segmentation
By Device Type
By Application
By
Deployment Type
By Country
Company Profiles
The List of Companies - North America Software as a Medical Device Market
The North America Software as a Medical Device Market is valued at US$ 8,353.94 Million in 2019, it is projected to reach US$ 39,854.20 Million by 2027.
As per our report North America Software as a Medical Device Market, the market size is valued at US$ 8,353.94 Million in 2019, projecting it to reach US$ 39,854.20 Million by 2027. This translates to a CAGR of approximately 22.2% during the forecast period.
The North America Software as a Medical Device Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Software as a Medical Device Market report:
The North America Software as a Medical Device Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Software as a Medical Device Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Software as a Medical Device Market value chain can benefit from the information contained in a comprehensive market report.