The market's growth is attributed to key driving factors, such as the rising importance of medical waste minimization and the growing use of reprocessed single-use devices. The limitations of reprocessed medical devices and stringent regulatory requirements are the factors restraining the market growth.
Reprocessing is a common practice worldwide, where hospitals prefer to reprocess single-use devices to minimize medical waste. About 45% of hospitals have reprocessing agreements with third-party reprocessing medical device companies in the US. Various regional regulatory authorities regulate reprocessing practices.
According to studies, reusing medical devices are a greener initiative, as it produces less medical waste, which is beneficial to the environment. Medical waste clogs landfills and isexpensive to avail bio waste disposal services to clear out. In comparison to the disposal of conventional solid waste, regulated medical waste (RMW) costs about 5–10 times higher, resulting in an increase in expenditure. Various health care practitioners noted that reusable and single-use devices are nearly comparable and proper device reprocessing has no negative consequences for consumers. As a result, the rising hospital awareness is aiding the growth of the reprocessed medical device market.
RMW, often known as "red bag waste," is a waste expense that typically costs hospitals 6–10 times more than conventional solid waste to dispose of the waste material. With growing initiatives, 95% of the devices are reprocessed annually rather than sent to landfills at the end of their life cycle. Stainless steel, aluminum, titanium, gold, polycarbonate, and polyurethane are the other re-processable raw materials in a hospital's RMW. Some hospitals have diverted more than 8,000 pounds of RMW from landfills each year by reprocessing, while bigger systems can recycle more than 50,000 pounds.
Also, the COVID-19 pandemic increased the amount of medical waste, which needs immediate attention. According to the new WHO report, the response to the COVID-19 pandemic has put tens of thousands of tons of extra medical waste on health care waste management systems worldwide, posing a serious threat to human and environmental health and highlighting the urgent need to improve waste management practices. According to WHO, The Global Analysis of Health Care Waste in the Context of COVID-19 was based on the approximately 87,000 metric tonnes of personal protective equipment (PPE) that was procured and shipped to support countries' urgent COVID-19 response needs through a joint UN emergency initiative between March 2020 and November 2021.. Therefore, the rising importance of medical waste minimization is driving the growth of the reprocessed medical devices market.
NORTH AMERICA REPROCESSED MEDICAL DEVICES MARKET SEGMENTATION
The North America reprocessed medical devices market, based on product, has been segmented into cardiovascular medical devices, gastroenterology biopsy forceps, orthopedic external fixation devices, laparoscopic medical devices, general surgery medical devices, non-invasive devices, and others. The North America reprocessed medical devices market, based on end user was segmented into hospitals and clinics, ambulatory surgical centers, medical laboratories, and others. Geographically, North America reprocessed medical devices market can be divided into U.S, Canada and Mexico.
Medline Industries, Inc., Arjo Medical Devices, Stryker Corporation, Teleflex Incorporated, Innovative Health, Johnson and Johnson Services, Inc., 3M, STERIS plc., Cardinal Health Inc and Vanguard AG are among the leading companies operating in the North America reprocessed medical devices market.
Strategic insights for the North America Reprocessed Medical Devices provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
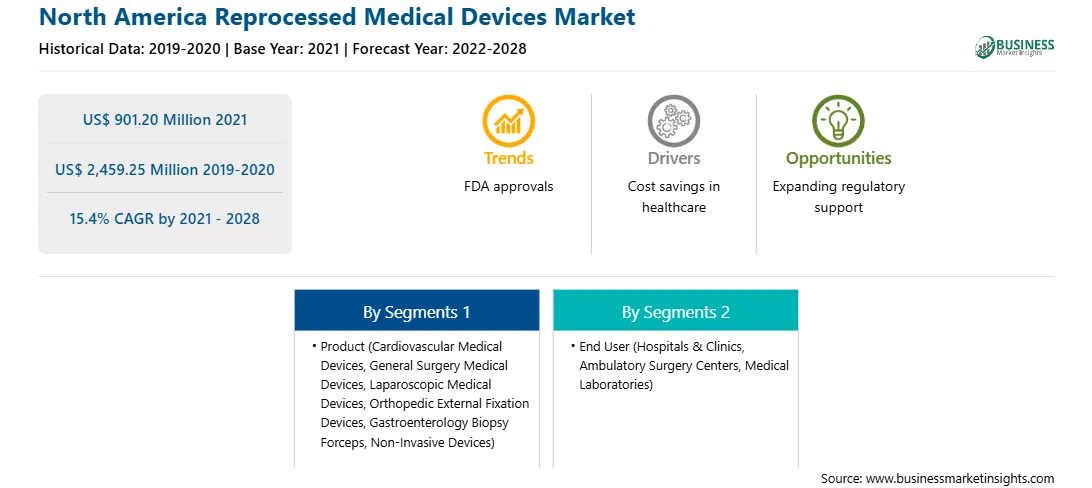
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 901.20 Million |
| Market Size by 2028 | US$ 2,459.25 Million |
| Global CAGR (2021 - 2028) | 15.4% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America Reprocessed Medical Devices refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The North America Reprocessed Medical Devices Market is valued at US$ 901.20 Million in 2021, it is projected to reach US$ 2,459.25 Million by 2028.
As per our report North America Reprocessed Medical Devices Market, the market size is valued at US$ 901.20 Million in 2021, projecting it to reach US$ 2,459.25 Million by 2028. This translates to a CAGR of approximately 15.4% during the forecast period.
The North America Reprocessed Medical Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Reprocessed Medical Devices Market report:
The North America Reprocessed Medical Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Reprocessed Medical Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Reprocessed Medical Devices Market value chain can benefit from the information contained in a comprehensive market report.