Market Introduction
Point-of-care molecular diagnostics include portable devices, and assays & kits used to detect and diagnose diseases in human samples, such as throat swab, blood, serum, and stool. Molecular diagnostics are shifting from centralized laboratories to decentralized point-of-care molecular testing. Due to its simplicity, convenience, rapid turnaround time, and potential to improve patient outcomes, POCT is rapidly gaining traction.
Moreover, the growing demand for specific viral detection methods that consume less time for timely infection control is expected to bolster the market growth during the forecast period.
The US has the highest number of COVID-19 cases of all countries in North America. This has negatively impacted various industries, and supply and distribution chains in the region. During the pandemic, life science companies shifted their focus in the development of novel drugs for the treatment of life-threatening diseases. In addition, the demand for rapid testing equipment has also increased, which is playing a prominent role in the growth of the North America point-of-care molecular diagnostics market. Moreover, continuous spread of COVID-19 is bolstering the demand for point-of-care molecular diagnostic kits. The adoption of these kits is boosting new product developments and launches. In March 2021, Eurofins' Clinical Enterprise, Inc. obtained an Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) for a direct-to-consumer (DTC) version of its EmpowerDX COVID-19 Home Collection Kit. Similarly, in July 2020, Clinical Diagnostics of Eurofins USA announced the availability of its pooled PCR test to detect SARS-CoV-2, which would substantially lower the cost per PCR test for clients.
Market Overview and Dynamics
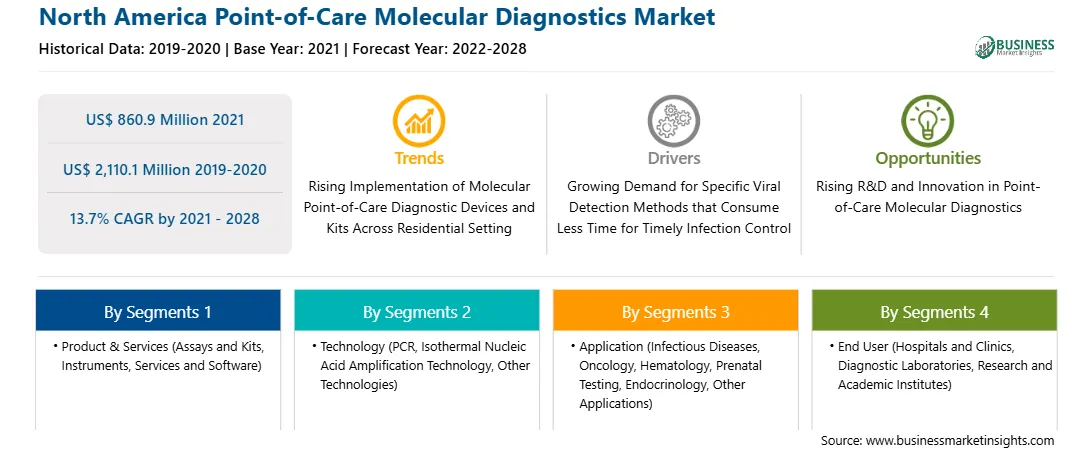
The North America Point-of-Care Molecular Diagnostics market is expected to reach US$ 2,110.1 million by 2028 from US$ 860.9 million in 2021. The market is estimated to grow at a CAGR of 13.7% from 2021–2028. The ongoing COVID-19 pandemic has also created opportunities for the manufacturers of POC molecular diagnostic kits for the detection of the novel coronavirus. For instance, ABBOTT, a global healthcare and medical equipment manufacturer, launched ID NOW. It is a molecular point-of-care instrument to detect new coronavirus infection in less than five minutes. ID NOW is a rapid, instrument-based, isothermal technique for detecting infectious illnesses qualitatively. Its proprietary isothermal nucleic acid amplification product & services generates molecular data in seconds, allowing doctors to make evidence-based treatment decisions during a patient visit. The ID NOW has not been certified or approved by the Food and Drug Administration (FDA). It has been approved by the FDA to use in authorized laboratories and patient care settings under an Emergency Use Authorization (EUA). Such innovations and product developments are expected to provide lucrative opportunities for the growth of the North America point-of-care molecular diagnostics market during the forecast period.
Key Market Segments
In terms of product & services, the assays and kits segment accounted for the largest share of the North America Point-of-Care Molecular Diagnostics market in 2020. In terms of technology, the PCR segment accounted for the largest share of the North America Point-of-Care Molecular Diagnostics market in 2020. In terms of application, the infectious diseases segment accounted for the largest share of the North America Point-of-Care Molecular Diagnostics market in 2020. In terms of end user, the diagnostic laboratories segment accounted for the largest share of the North America Point-of-Care Molecular Diagnostics market in 2020.
Major Sources and Companies Listed
A few major primary and secondary sources referred to for preparing this report on the Point-of-Care Molecular Diagnostics market in North America are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are bioMérieux SA, F. Hoffmann-La Roche Ltd., Danaher Corporation, Enzo Biochem, Inc., Abbott, binx health, Inc., Meridian BioScience, Inc., Biocartis, Quidel Corporation, and Bio-Rad Laboratories, Inc.
Reasons to Buy Report
NORTH AMERICA POINT-OF-CARE MOLECULAR DIAGNOSTICS MARKET SEGMENTATION
By Technology
By Application
By End User
By Country
Companies Mentioned
Strategic insights for the North America Point-of-Care Molecular Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 860.9 Million |
| Market Size by 2028 | US$ 2,110.1 Million |
| Global CAGR (2021 - 2028) | 13.7% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product & Services
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America Point-of-Care Molecular Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The North America Point-of-Care Molecular Diagnostics Market is valued at US$ 860.9 Million in 2021, it is projected to reach US$ 2,110.1 Million by 2028.
As per our report North America Point-of-Care Molecular Diagnostics Market, the market size is valued at US$ 860.9 Million in 2021, projecting it to reach US$ 2,110.1 Million by 2028. This translates to a CAGR of approximately 13.7% during the forecast period.
The North America Point-of-Care Molecular Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Point-of-Care Molecular Diagnostics Market report:
The North America Point-of-Care Molecular Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Point-of-Care Molecular Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Point-of-Care Molecular Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.