North America is the largest market for point of care diagnostics. The region includes US, Canada, and Mexico. The growth of the North America market is characterized by the growing prevalence of infectious diseases, government support for advanced diagnostics kits and instruments, and increasing efforts in R&D activities undertaken by the market players operating in the market. In addition, technological advancements in the diagnosis industry is likely to be a major growth stimulator for the point of care diagnostics market in North America. In US, Centers for Medicare and Medicaid Services (CMS) directs Medicare, which is a federal program for adults aged above 65 and people with disabilities, and it works along with state governments. CMS maintain both Medicaid and the Children’s Health Insurance Program (CHIP), an assembly of federal-state programs for various low-income people. Medicare delivers coverage for physician services, hospitalization, and a voluntary supplementary program, prescription drugs. Additionally, the presence of top revenue-generating medical device companies that can invest a significant amount in R&D supports the market growth as these companies manufacture innovative medical devices.
In case of COVID-19, North America is highly affected specially the US. The outbreak of the COVID-19 pandemic situation shown some favorable scenario for players operating in the point of care diagnostics market. Significant population of North America is infected with coronavirus. For instance, the US is a highly affected country in the North American regions. The effect of the corona is mild in adults; however, it is adverse in older people. The infection of corona among older people has resulted in severe complications and has accounted for a good number of deaths in the country. But during the pandemic situation, life science companies engaged in development of novel drugs for the treatment of life threaten diseases. In addition, increasing demand for rapid testing equipment is also playing a prominent role in the growth of the North America point of care diagnostics market. Furthermore, due to the rising numbers of COVID-19 population has positive impact on the adoption of point of care diagnostic kits. This adoption is boosting new product development and launches in the market. For instance, in July 2020, Eurofins U.S. Clinical Diagnostics has announced the availability of its pooled PCR test to detect SARS-CoV-2, which would substantially lower the cost per PCR test for clients. Moreover, in March 2020, Abbott received FDA approval for its molecular point-of-care test for the diagnosis of COVID-19. The test is capable to deliver positive results in five minutes and negative result in less than fifteen minutes. Such product launches are expected to accelerate the growth of the North America point of care diagnostics market.
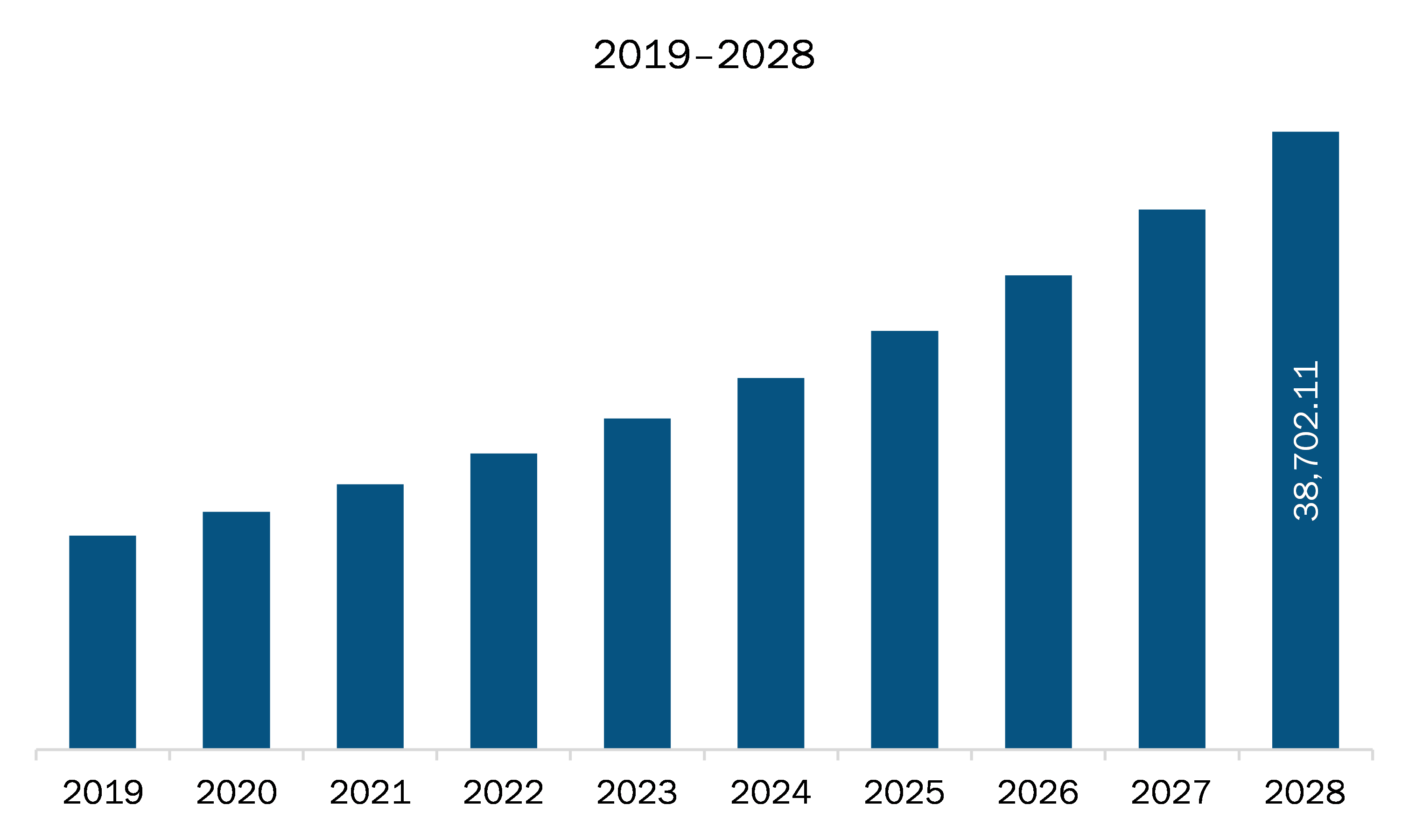
Strategic insights for the North America Point of Care Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
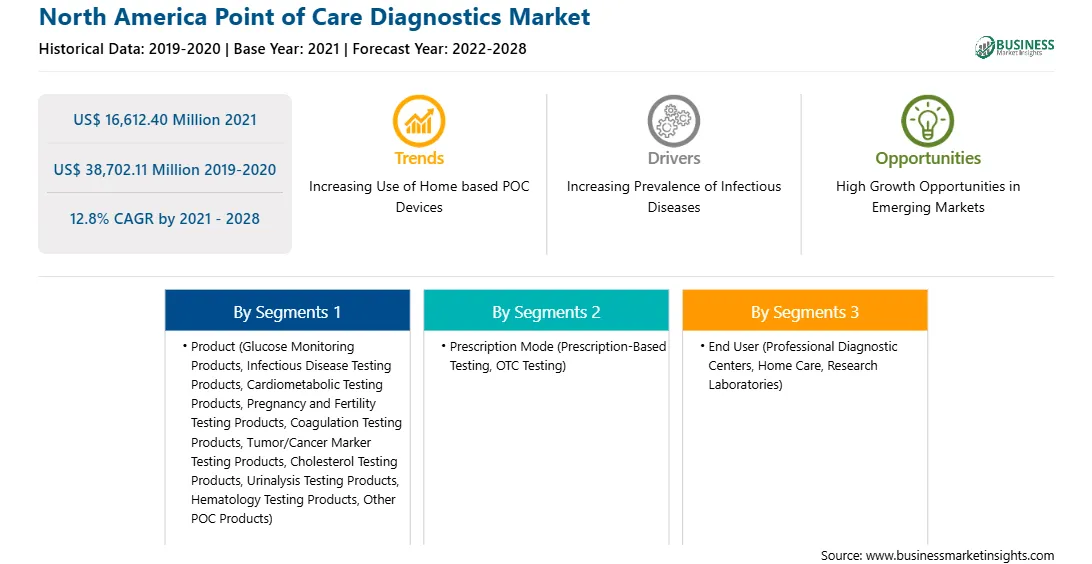
| Market size in 2021 | US$ 16,612.40 Million |
| Market Size by 2028 | US$ 38,702.11 Million |
| Global CAGR (2021 - 2028) | 12.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America Point of Care Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The North America point of care diagnostics market is expected to grow from US$ 16,612.40 million in 2021 to US$ 38,702.11 million by 2028; it is estimated to grow at a CAGR of 12.8% from 2021 to 2028. Various conferences and events are held to make people aware about devices and give overview of the common key principles that should be applied when establishing and maintaining point-of-care testing (POCT) services. Participants in these conferences/events can utilize these learnings to develop a framework for introducing POCT for a specific clinical need relevant to their family practice. In June 2019, SELECTBIO held the Point-of-Care, Biosensors & Mobile Diagnostics conference. The Emerging Trends and Themes in Point-of-Care Diagnostics and Mobile Diagnostics, Commercialization of Microfluidic Devices for Point-of-Care Applications, and Paper-based Analytical Devices for the Diagnosis and Monitoring of Infectious Diseases at the Point of Care were the major topics covered in this conference. So, burgeoning number of conferences and events is expected to escalate the market growth.
In terms of product, the glucose monitoring products segment accounted for the largest share of the North America point of care diagnostics market in 2020. In terms of prescription mode, the prescription-based testing segment held a larger market share of the North America point of care diagnostics market in 2020. Further, the professional diagnostic centers segment held a larger share of the North America point of care diagnostics market based on end user in 2020.
A few major primary and secondary sources referred to for preparing this report on the North America point of care diagnostics market are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Abbott; BD; bioMerieux SA; BIO-RAD LABORATORIES INC.; Danaher; F. HOFFMANN-LA ROCHE LTD.; Johnson and Johnson Services, Inc.; Nova Biomedical; Polymer Technology Systems, Inc. (PTS); and Siemens AG.
The North America Point of Care Diagnostics Market is valued at US$ 16,612.40 Million in 2021, it is projected to reach US$ 38,702.11 Million by 2028.
As per our report North America Point of Care Diagnostics Market, the market size is valued at US$ 16,612.40 Million in 2021, projecting it to reach US$ 38,702.11 Million by 2028. This translates to a CAGR of approximately 12.8% during the forecast period.
The North America Point of Care Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Point of Care Diagnostics Market report:
The North America Point of Care Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Point of Care Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Point of Care Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.