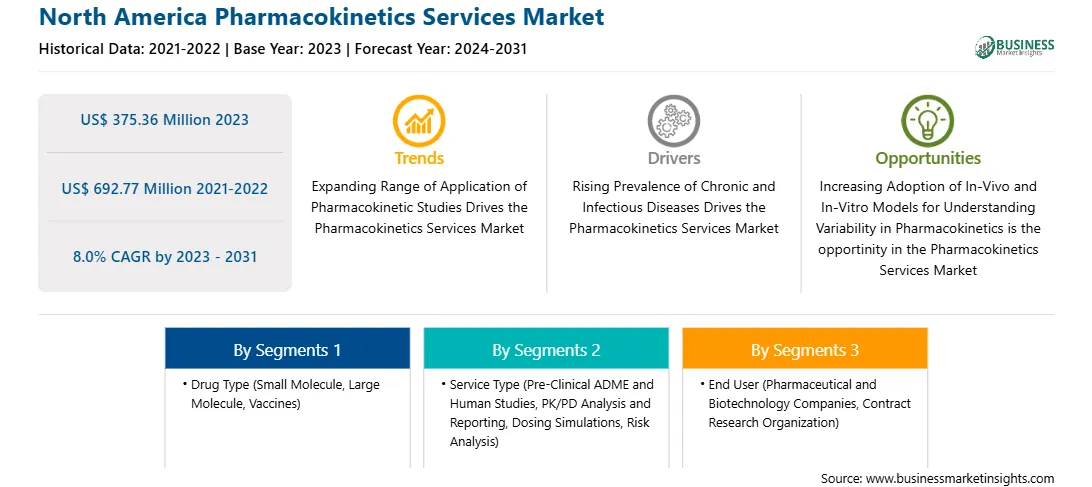
The North America pharmacokinetics services market was valued at US$ 375.36 million in 2023 and is expected to reach US$ 692.77 million by 2031; it is estimated to register a CAGR of 8.0% from 2023 to 2031.
Pharmacokinetic studies are being employed to determine several parameters such as human-equivalent doses (HED) level, no-observed effect level (NOEL), and pharmacokinetic/pharmacodynamic testing. The main focus of any pre-clinical program conducted is to support the analysis of a safe and effective dose range for testing in pharmacokinetic studies. Several toxicology studies conducted data on the NOEL, i.e., the highest dose that does not produce adverse effects. This dose level is further converted to a HED level on a comparative body-surface area basis if there is lack of clinical pharmacokinetic data. Understanding the pharmacokinetic profile of a potential drug candidate plays an important role in the drug discovery program. Regulatory authorities play a major role propelling the implementation of pharmacokinetic studies. These studies are also instrumental in optimizing pharmaceutical care services provided to patients admitted in hospitals. The PK model is often applied to determine exposure to drugs, concentrations of drugs to analyze optimal dosage, and analyze the disposition of drugs in the human body. Healthcare providers implement the principles of PK to design monitor drug concentrations, doses of some drugs, maximize the intended therapeutic outcomes and minimize toxicities.
The US accounts for the largest share of the North America pharmacokinetics services market in North America. The country has emerged as a leading clinical research destination; it accounts for ~50% of the total clinical trials conducted in the world. The availability of established medical infrastructure, fast approval timelines, and favorable regulatory framework create a conducive environment for pharmaceutical research companies to conduct clinical trials. Moreover, the data generated in trials conducted in the US is accepted globally. As per a World Health Organization (WHO) report, the US registered the highest number of clinical trials (157,618) in 2021.
Strategic insights for the North America Pharmacokinetics Services provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the North America Pharmacokinetics Services refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.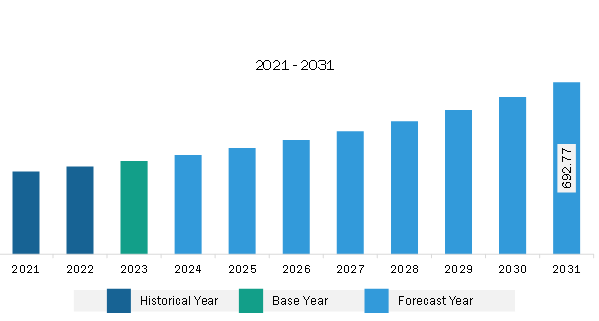
North America Pharmacokinetics Services Strategic Insights
North America Pharmacokinetics Services Report Scope
Report Attribute
Details
Market size in 2023
US$ 375.36 Million
Market Size by 2031
US$ 692.77 Million
Global CAGR (2023 - 2031)
8.0%
Historical Data
2021-2022
Forecast period
2024-2031
Segments Covered
By Drug Type
By Service Type
By End User
Regions and Countries Covered
North America
Market leaders and key company profiles
North America Pharmacokinetics Services Regional Insights
The North America pharmacokinetics services market is categorized into drug type, service type, therapeutic application, end user, and country.
Based on drug type, the North America pharmacokinetics services market is segmented small molecule, large molecule, and vaccines. The small molecule segment held the largest market share in 2023.
In terms of service type, the North America pharmacokinetics services market is categorized into pre-clinical ADME and human studies, PK/PD analysis and reporting, dosing simulations, risk analysis, and others. The pre-clinical ADME and human studies segment held the largest market share in 2023.
By therapeutic application, the North America pharmacokinetics services market is segmented into oncology, infectious diseases, neurological disorders, autoimmune diseases, gynaecological disorders, cardiovascular diseases, respiratory disorders, and others. The oncology segment held the largest market share in 2023.
By end user, the North America pharmacokinetics services market is segmented into pharmaceutical and biotechnology companies, contract research organization, and others. The contract research organization segment held the largest market share in 2023.
By country, the North America pharmacokinetics services market is segmented into the US, Canada, and Mexico. The US dominated the North America pharmacokinetics services market share in 2023.
Charles River Laboratories International Inc; Eurofins Scientific SE; Evotec SE; Certara Inc.; Parexel International Corp; Thermo Fisher Scientific Inc.; Allucent; PACIFIC BIOLABS; and SGS SA are some of the leading companies operating in the North America pharmacokinetics services market.
The North America Pharmacokinetics Services Market is valued at US$ 375.36 Million in 2023, it is projected to reach US$ 692.77 Million by 2031.
As per our report North America Pharmacokinetics Services Market, the market size is valued at US$ 375.36 Million in 2023, projecting it to reach US$ 692.77 Million by 2031. This translates to a CAGR of approximately 8.0% during the forecast period.
The North America Pharmacokinetics Services Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Pharmacokinetics Services Market report:
The North America Pharmacokinetics Services Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Pharmacokinetics Services Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Pharmacokinetics Services Market value chain can benefit from the information contained in a comprehensive market report.