The North America GMP testing service market is expected to grow from US$ 846.02 million in 2022 to US$ 1,260.96 million by 2028; it is estimated to grow at a CAGR of 6.9% from 2022 to 2028.
Flourishment of Medical Device Industry Bolsters North America GMP Testing Service Market Growth
The medical devices industry is contributing to the development of medical technologies that have the potential to reinforce the diagnosis and treatment of various illnesses and support the effective manufacturing of medical devices. The equipment produced by the medical devices industry range from common supplies such as surgical masks to high-tech imaging equipment. Medical devices such as insulin delivery systems, glucose monitors, oxygen concentrators, and nebulizers are increasingly being used for the at-home diagnosis and treatment of a few medical disorders. These devices are integrated with the patient’s EMR, wearables, cellphones, and telehealth platforms to provide critical insights for enhanced clinical and operational decision-making. According to data provided by PRODSMART, the medical devices manufacturing industry was valued at ~US$ 4.2 billion in 2020. A rise in the incidence of chronic diseases and the growth of the geriatric population are the major factors that are expected to influence the progress of the medical device industry in the coming years. The operations of medical devices with Internet of Medical Things (IoMT) systems are facilitated by smart gadgets, smart sensors, and other lightweight communication devices. Such innovations assist healthcare companies in improving patient outcomes, lowering costs, and increasing efficiency. The GMPs are enormously important in the manufacturing of medical devices because they ensure a level of quality, safety, purity, and strength in any products that are released to the public. Adhering to these practices helps manufacturers in preventing adverse effects, product recalls, and defects, which may lead to costly liability lawsuits in the future. As quality control and consistent testing environments are pivotal for a successful manufacturing cycle, the focus on GMPs is increasing for avoiding product contamination, failure, and quality deviation. Thus, by establishing a strong system of well-regulated operational management led by GMPs, a medical device manufacturer can develop vast volumes of quality products, along with reducing costly delays. Thus, the above-mentioned factors are bolstering the growth of North America GMP testing service market.
North America GMP Testing Service Market Overview
The North America GMP testing service market is segmented into the US, Canada, and Mexico. The US held the largest share of the market in 2021. The growing pharmaceutical industry, and the increasing need for developing novel drugs and medical devices are accelerating the growth of the GMP testing service market in North America. Moreover, the rising health expenditure by governments and an increase in outsourcing activities for the quality assurance of medical products are propelling the expansion of this market in this region. The US holds a significant share of the North America GMP testing service market. The market growth in this country is attributed to rising healthcare expenditure by the government, ongoing developments in the pharmaceuticals industry, and rising demand for novel drugs owing to various infectious diseases.
North America GMP Testing Service Market Revenue and Forecast to 2028 (US$ Million)
Strategic insights for the North America Needle-Free Injection Systems provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the North America Needle-Free Injection Systems refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.North America Needle-Free Injection Systems Strategic Insights
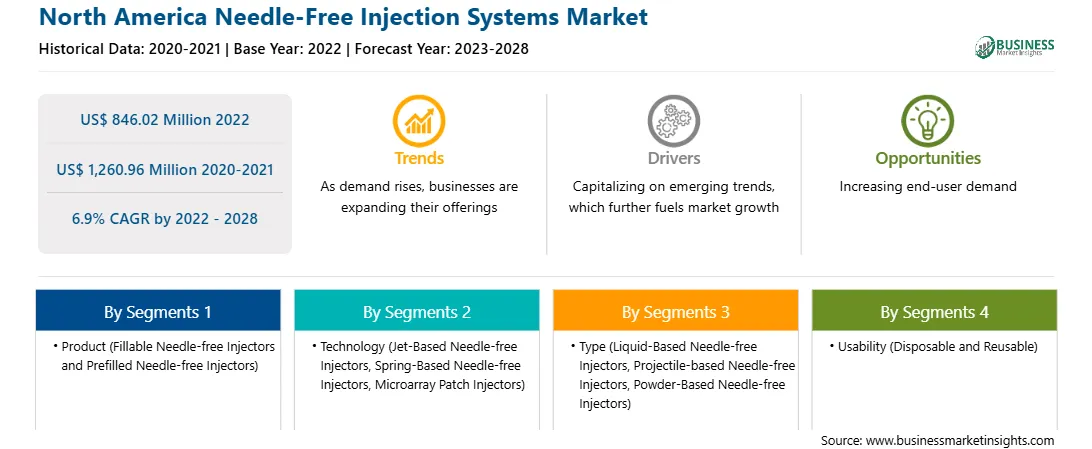
North America Needle-Free Injection Systems Report Scope
Report Attribute
Details
Market size in 2022
US$ 846.02 Million
Market Size by 2028
US$ 1,260.96 Million
Global CAGR (2022 - 2028)
6.9%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Product
By Technology
By Type
By Usability
By Site of Delivery
Regions and Countries Covered
North America
Market leaders and key company profiles
North America Needle-Free Injection Systems Regional Insights
North America GMP Testing Service Market Segmentation
The North America GMP testing service market is segmented on the basis of service type, end user, and country. Based on service type, the market is segmented into product validation testing, bioanalytical services, packaging & shelf life testing, and other services type. The product validation testing segment held the largest market share in 2022.
Based on end user, the North America GMP testing service market is bifurcated into pharmaceutical and biopharmaceutical companies, and medical device companies. The pharmaceutical and biopharmaceutical companies segment held a larger market share in 2022.
The North America GMP testing service market, by country, is segmented into the US, Canada, and Mexico. The US dominated the market share in 2022.
Eurofins Scientific; Almac Group; INTERTEK GROUP PLC; WuXi AppTec; Sartorius AG; North American Science Associates, Inc.; Nelson Laboratories, LLC.; Boston Analytical; Pace Analytical Services; and Thermo Fisher Scientific Inc. (PPD Inc.) are among the leading companies operating in the North America GMP testing service market.
The North America Needle-Free Injection Systems Market is valued at US$ 846.02 Million in 2022, it is projected to reach US$ 1,260.96 Million by 2028.
As per our report North America Needle-Free Injection Systems Market, the market size is valued at US$ 846.02 Million in 2022, projecting it to reach US$ 1,260.96 Million by 2028. This translates to a CAGR of approximately 6.9% during the forecast period.
The North America Needle-Free Injection Systems Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Needle-Free Injection Systems Market report:
The North America Needle-Free Injection Systems Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Needle-Free Injection Systems Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Needle-Free Injection Systems Market value chain can benefit from the information contained in a comprehensive market report.