North America is the largest market for the micro catheters and micro guidewires, with the US holding the largest market share, followed by Canada. The micro catheters and micro guidewires market is likely to experience significant growth in the US due to the rising prevalence of the cardiac conditions among the people. As per the American Heart Association's "Heart Disease and Stroke Statistics 2019”, around 121.5 million adults in the US are living with cardiovascular disease, and every year 805,000 Americans suffer from a heart attack. Additionally, according to the CDC, about 795,000 people in the U.S. suffer from cerebrovascular stroke every year. Therefore, the cost of the treatments increases for the patients. The indirect and direct cost associated with the total cardiovascular diseases and stroke are anticipated to entire more than US$ 329.7 billion which includes both health expenditures and productivity loss. Additionally, across the country heart disease and stroke are the first and fifth leading cause of death. Hypertension is major risk factor for the heart disease and stroke and is expensive in terms of lives lost and health outcomes, however it is greatly treatable. According to the Centers for Disease Control and Prevention statistics, in 2018 approximately 75 million adults across US lived with high blood pressure. The prevalence is expected to rise in the coming future and therefore, it is anticipated that the incidences of the cardiovascular is likely to increase in future. Also, the US is among the highly advanced countries which have various technologies available for the medical devices, the country is also involved in the research and development of the medical technologies. Significantly increasing prevalence of cardiovascular diseases is the major factor driving the growth of North America micro catheters and micro guidewires market.
COVID-19 virus outbreak was first observed in December 2019 in Wuhan (China), and it has spread to ~100 countries across the world, with the World Health Organization (WHO) stating it as a public health emergency. The global impacts of COVID-19 are being felt across several markets, including the market for microcatheters and micro guidewires market.
Strategic insights for the North America Micro Catheters and Micro Guidewires provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
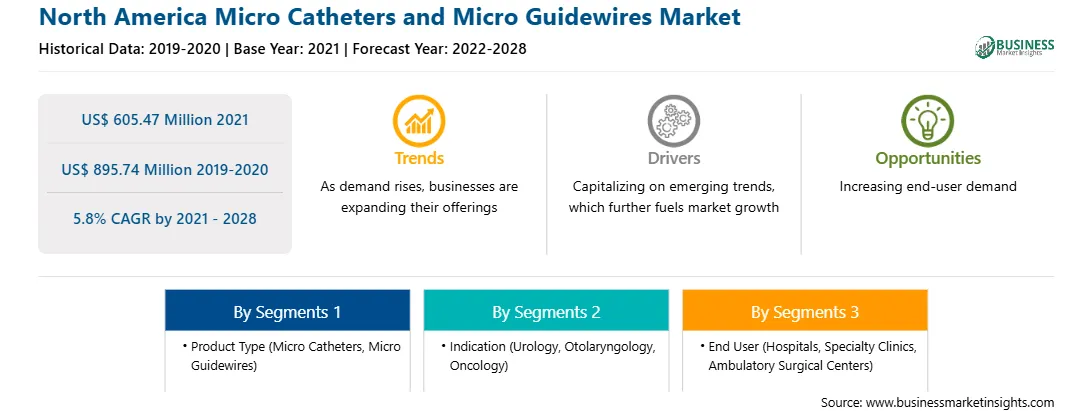
| Market size in 2021 | US$ 605.47 Million |
| Market Size by 2028 | US$ 895.74 Million |
| Global CAGR (2021 - 2028) | 5.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America Micro Catheters and Micro Guidewires refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
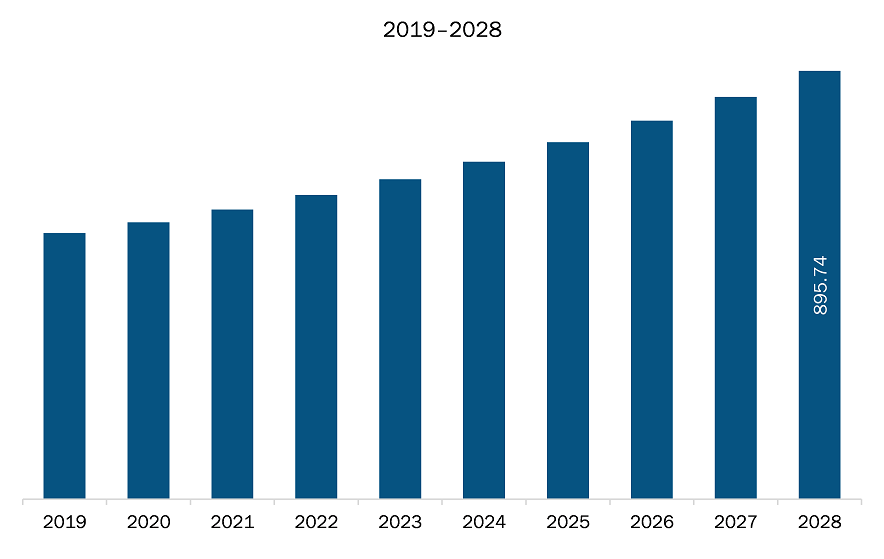
The micro catheters and micro guidewires market in North America is expected to grow from US$ 605.47 million in 2021 to US$ 895.74 million by 2028; it is estimated to grow at a CAGR of 5.8% from 2021 to 2028. Growing number of product approvals and launches; Extended functionalities and technologies incorporated in new devices are contributing to the rise in demand for micro catheters and micro guidewires. In March 2021, Transit Scientific received CE approval for its XO CrossO Microcatheter platform. The new microcatheter incorporates novel non-tapered design, which offers better functionality. Moreover, the new platform delivers better torque response, trackability, and new pushability levels during a surgical procedure. Further, in January 2021, Baylis Medical announced the launch of JLL electrophysiology microcatheter in the Europe market. The introduction of catheter enables physicians to reach complex and inaccessible areas in a heart. Further, in June 2020, Insight Lifetech received CE approval for its TruePhysio Rapid Exchange FFR microcatheter. The newly launched catheter is compatible with any 0.014’’ guidewire for accurate procedures. Also, in May 2019, Embolx, Inc. introduced Sniper K-tip microcatheter under sniper balloon occlusion microcatheter. This newly introduced microcatheter is especially designed for an arterial embolization procedure. Furthermore, in August 2020, Scientia Vascular received FDA approval for its Zoom Wire 14 guidewire. The new device incorporates capabilities to gain access of nervous system during hemorrhagic and ischemic stroke and other associated vascular procedures. Thus, the growing number of product launches and approvals are estimated to offer lucrative opportunities for the growth of the micro catheter and micro guidewires market during the forecast period. This is bolstering the growth of the micro catheters and micro guidewires market.
In terms of product type, micro catheter segment held the largest share of the market in 2020, and the same segment is anticipated to register the highest CAGR in the market during the forecast period. In terms of indication type, cardiology segment held the largest share of the market in 2020, however, the oncology segment is anticipated to register the highest CAGR in the market during the forecast period. Similarly, based on end user, hospitals segment held the largest share of the market in 2020 and is estimated to register the highest CAGR in the market during the forecast period.
A few major primary and secondary sources referred to for preparing this report on the micro catheters and micro guidewires market in North America are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are B. Braun Melsungen AG; BD
The North America Micro Catheters and Micro Guidewires Market is valued at US$ 605.47 Million in 2021, it is projected to reach US$ 895.74 Million by 2028.
As per our report North America Micro Catheters and Micro Guidewires Market, the market size is valued at US$ 605.47 Million in 2021, projecting it to reach US$ 895.74 Million by 2028. This translates to a CAGR of approximately 5.8% during the forecast period.
The North America Micro Catheters and Micro Guidewires Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Micro Catheters and Micro Guidewires Market report:
The North America Micro Catheters and Micro Guidewires Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Micro Catheters and Micro Guidewires Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Micro Catheters and Micro Guidewires Market value chain can benefit from the information contained in a comprehensive market report.