A laboratory developed test (LDT) is a type of in vitro diagnostic test that is designed and used within a single laboratory. These tests can be utilized to estimate or distinguish an extensive assortment of analytes materials such as proteins, chemical compounds like glucose or cholesterol, or DNA, from a specimen received from human anatomy. The expansion of automated in vitro diagnostics (IVD) methods for labs and dispensaries to render precise, and error-free analysis is anticipated to fuel the increment.
Strategic insights for the North America Laboratory Developed Test provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
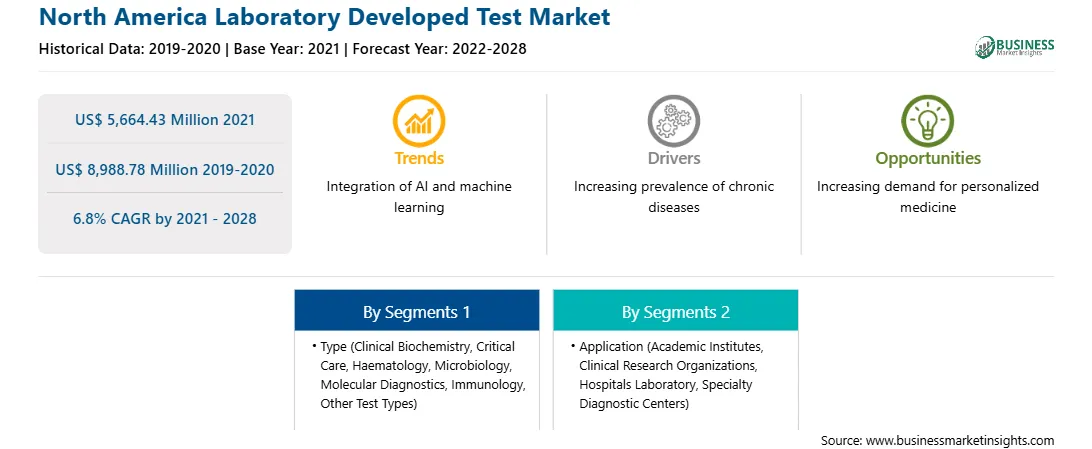
| Market size in 2021 | US$ 5,664.43 Million |
| Market Size by 2028 | US$ 8,988.78 Million |
| Global CAGR (2021 - 2028) | 6.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America Laboratory Developed Test refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
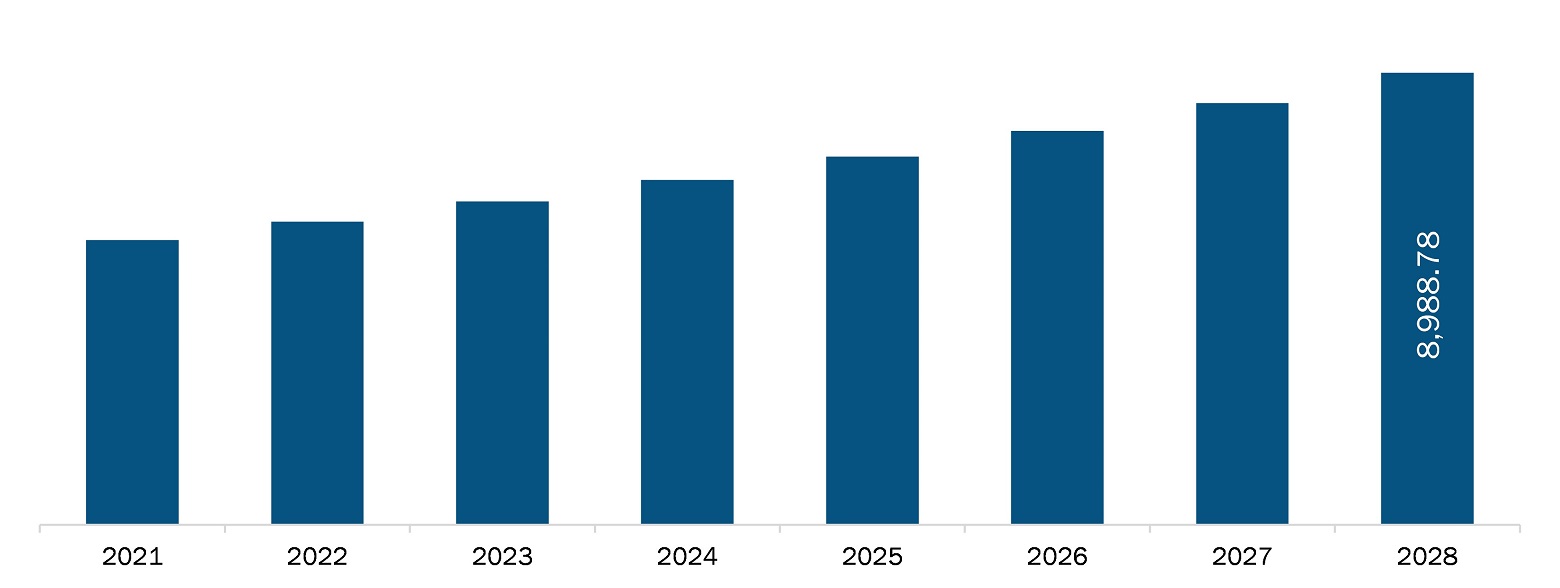
The North America laboratory developed test market is expected to reach US$ 8,988.78 million by 2028 from an estimated value of US$ 5,664.43 million in 2021; it is likely to register a CAGR of 6.8% from 2021 to 2028. The key factors driving the growth of market are increasing incidence of cancer and genetic disorders and a large number of product launches for laboratory developed tests. However, the changing regulatory landscape may hamper the growth of the North America laboratory development test market during the forecast period.
Cancer is one of the leading causes of death worldwide. As per the WHO estimates, cancer was a cause of ~9.6 million deaths in 2018. Clinical diagnostics helps in detecting early signs and risk factors, paving way for early intervention. The laboratory developed tests (LDTs) have a decisive impact on each step of diagnosis, from screening to the prevention of certain diseases and early diagnosis at the onset of illness. For instance, the Oncotype DX lab test is used to determine if chemotherapy would benefit patients with early-stage breast cancer. It also helps evaluate the likelihood of disease recurrence; for this, the test is performed on a small tissue sample removed during breast cancer surgery. In addition, the increasing incidence of genetic disorders is also driving the demand for LDTs. A large number of LDTs are available for genetic testing owing to a lack of availability of genetic tests in the market. As per the Association for Molecular Pathology 2018 assessment, ~70,000 genetic tests are available in the medical market, and most of these are LDTs. Therefore, the rising incidence of cancer and growing awareness regarding the importance of early diagnostics are boosting the adoption of LDTs. Also, LDTs are developed and used within laboratories and are not distributed or sold to other laboratories or healthcare facilities. These tests are developed to overcome the challenge of the unavailability of commercial tests. Many LDTs are genetic tests developed for rare diseases. Thus, the frequency of development and introduction of new LDTs is high. Also, the increasing emergence of SARS-CoV-2 variants has highlighted the need to identify, trace, and track mutations across the complete viral genome. Hence, increasing research on the development of LDTs to detect cancer and autoimmune diseases is driving the market growth.
The COVID-19 pandemic has emphasized a need for widespread diagnostic testing. The commercially available test kits have proved insufficient to meet the demand owing to manufacturing, supply chain limitations, and the timeline required for Food and Drug Administration (FDA) review and authorization of test products. In contrast, laboratory developed tests (LDTs) are internally developed and validated at the performing laboratory allowed laboratories to fill the testing gaps during the pandemic and in other clinical scenarios where commercial tests are scarce. The government regulation efforts have also bolstered the laboratory developed tests growth since the pandemic. In August 2020, to address the COVID-19 testing shortage, the US Department of Health and Human Services (HHS) made a brief statement that effectively rescinded the FDA guidance requiring clinical labs to seek an emergency use authorization (EUA) or submit data to the FDA in conjunction with offering laboratory-developed tests (LDTs).A laboratory developed test (LDT) is a type of in vitro diagnostic test that is designed and used within a single laboratory. These tests can be utilized to estimate or distinguish an extensive assortment of analytes materials such as proteins, chemical compounds like glucose or cholesterol, or DNA, from a specimen received from human anatomy. The expansion of automated in vitro diagnostics (IVD) methods for labs and dispensaries to render precise, and error-free analysis is anticipated to fuel the increment.
The North America laboratory developed test market, by type, is segmented into clinical biochemistry, critical care, haematology, microbiology, molecular diagnostics, immunology, and other test types. The haematology segment is subsegmented into coagulation and hemostasis, hemoglobin testing, blood count testing, and others. The molecular diagnostics segment held the largest share of the market in 2021. However, the haematology segment is anticipated to register the highest CAGR during the forecast period.
The North America laboratory developed test market, by application, is segmented into academic institutes, clinical research organizations, hospitals laboratory, specialty diagnostic centers, and others. The hospitals laboratory segment held the largest share of the market in 2021. However, the specialty diagnostic centers segment is anticipated to register the highest CAGR during the forecast period.
A few of the primary and secondary sources associated with this report on the North America laboratory developed test market are the Centers for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), and American Cancer Society (ACS).
The North America Laboratory Developed Test Market is valued at US$ 5,664.43 Million in 2021, it is projected to reach US$ 8,988.78 Million by 2028.
As per our report North America Laboratory Developed Test Market, the market size is valued at US$ 5,664.43 Million in 2021, projecting it to reach US$ 8,988.78 Million by 2028. This translates to a CAGR of approximately 6.8% during the forecast period.
The North America Laboratory Developed Test Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Laboratory Developed Test Market report:
The North America Laboratory Developed Test Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Laboratory Developed Test Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Laboratory Developed Test Market value chain can benefit from the information contained in a comprehensive market report.