Market Introduction
The North America healthcare regulatory affairs outsourcing market is categorized into the US, Canada, and Mexico. North America is likely to capture significant share of the global market in 2021. The key factors that are driving the growth of the market are rising number of patent expirations along with growing costs of research and development activities. However, during the forecast period the market is likely to get restrained by the factors such as high fluctuations in price along with hidden expenses in the regulatory services delivered by diverse Clinical Research Organizations. The US is the largest market for healthcare regulatory affairs outsourcing at a global level. The market's growth is attributed to the enormous number of R&D activities in the field of drug discovery carried out in the country. The U.S. healthcare industry’s effort to minimize costs without compromising on quality of services is the major factor for the outsourcing of healthcare and medical services to other countries. The rigorous regulations imposed by the Health Insurance Portability and Accountability Act (HIPAA) of 1996 towards the establishment of national standards for electronic health care transactions and national identifiers for providers, health insurance plans, and employers has boosted the market growth. The standards and regulations are compulsory and require infrastructure which hospitals lack, thus making outsourcing unavoidable, and driving the growth of the U.S. healthcare outsourcing market. The rapid developments in the market and entry of global players into the market have resulted in partnerships, mergers & acquisitions, and joint ventures in the market. Moreover, the medical service vendors are mostly involved in making strategic partnerships with hospitals to provide outsourcing solutions. Rising regulatory pressure on healthcare companies is the major factor driving the growth of the North America healthcare regulatory affairs outsourcing market.
COVID-19 virus outbreak was first observed in December 2019 in Wuhan (China), and it has spread to ~100 countries across the world, with the World Health Organization (WHO) stating it as a public health emergency. The global impacts of COVID-19 are being felt across several markets. Although the healthcare sector had witnessed SARS, H1N1, and other outbreaks in the last few years, the severity of the COVID-19 has made the situation more complicated due to its mode of transmission. North America witnessed growing number of COVID-19 cases since its outbreak. For instance, according to Worldometer, the number of cases reached to 34,434,803 million with 617,875 deaths reported in the United States as of 23rd June 2021. Similar impact was noticed in Mexico and Canada. North America is one of the most important regions for the adoption and growth of new technologies due to flattering government policies to boost innovation, the presence of a huge industrial base, and high purchasing power, mainly in developed countries such as the US and Canada. Hence, any impact on the growth of industries is expected to affect the region's economic growth negatively. Presently, the US is the world’s worst-affected country due to the COVID-19 outbreak, with 28,659,480 confirmed cases and 520,751 deaths as per the WHO. The US is one of the eminent markets for healthcare outsourcing services. The factory and business shutdowns across the US, Canada, and Mexico negatively impacted various industries in 2020. However, COVID-19 has placed many regulatory and outsourcing teams under pressure but, it also has had a positive impact on the bio/pharmaceutical outsourcing industry, wherein the demand for R&D activity is increasing leading to a rise in regulatory affairs assistance. This rising demand has caused various CROs to focus on their outsourcing and other operations.
Strategic insights for the North America Healthcare Regulatory Affairs Outsourcing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the North America Healthcare Regulatory Affairs Outsourcing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.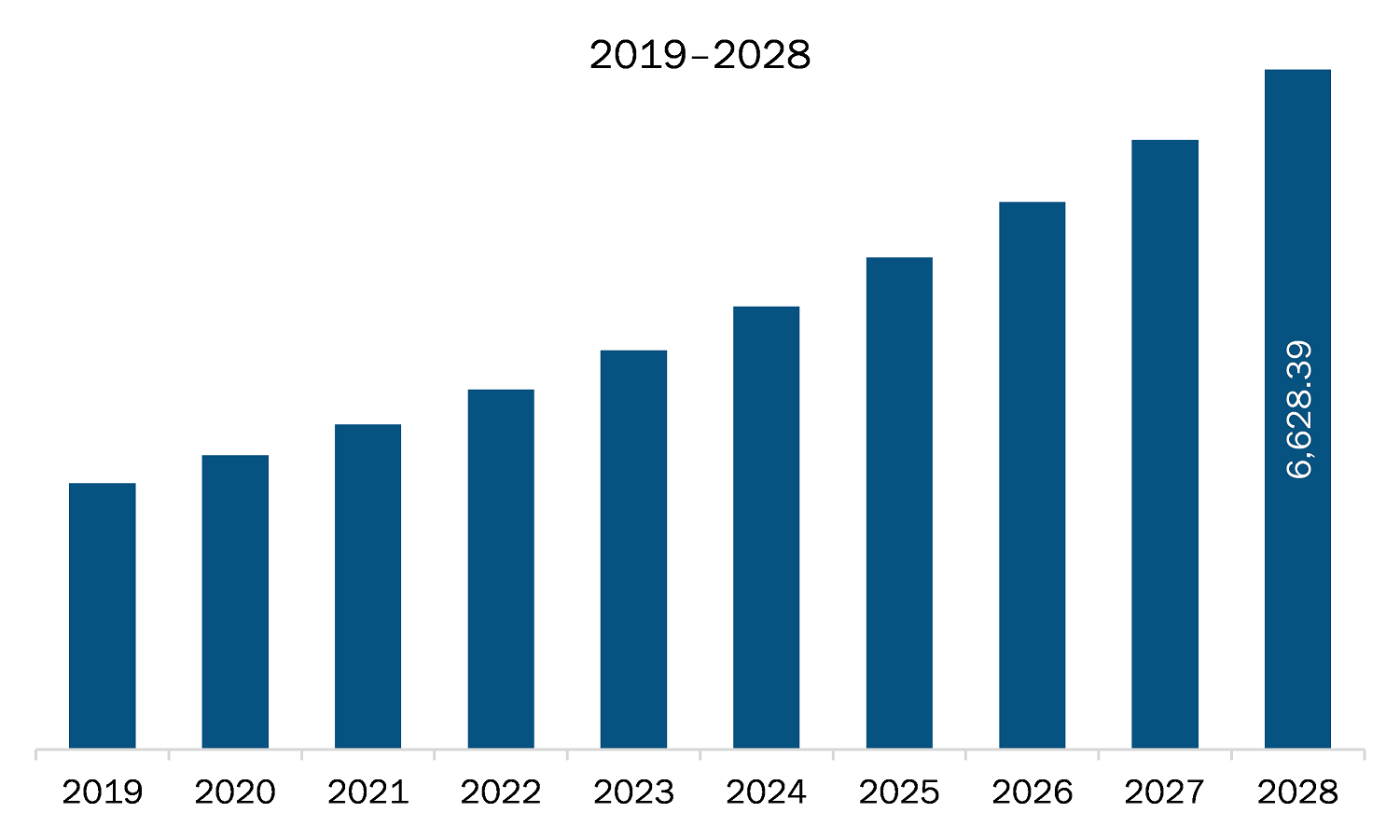
North America Healthcare Regulatory Affairs Outsourcing Strategic Insights
North America Healthcare Regulatory Affairs Outsourcing Report Scope
Report Attribute
Details
Market size in 2021
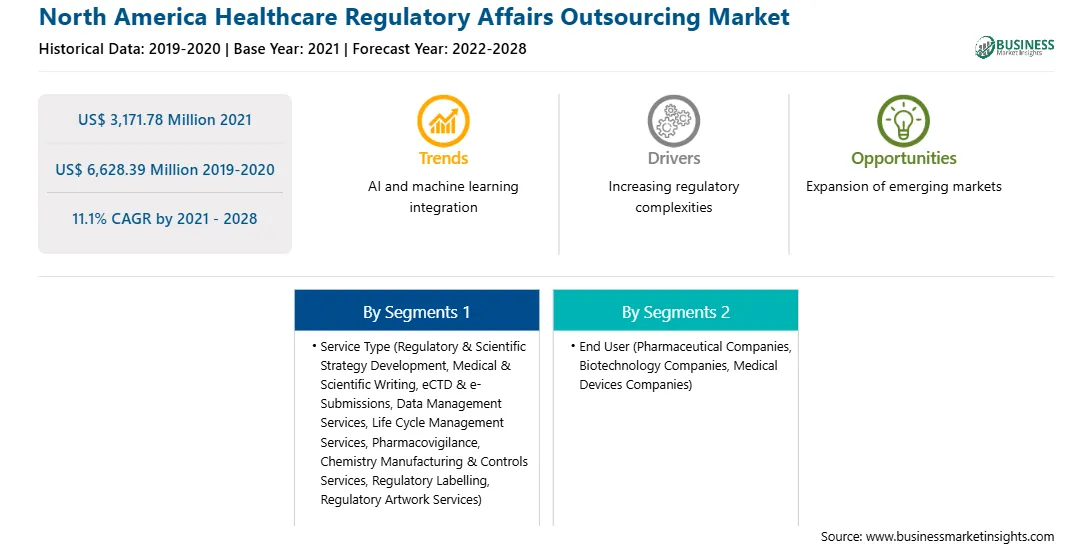
US$ 3,171.78 Million
Market Size by 2028
US$ 6,628.39 Million
CAGR (2021 - 2028) 11.1%
Historical Data
2019-2020
Forecast period
2022-2028
Segments Covered
By Service Type
By End User
Regions and Countries Covered
North America
Market leaders and key company profiles
North America Healthcare Regulatory Affairs Outsourcing Regional Insights
Market Overview and Dynamics
The healthcare regulatory affairs outsourcing market in North America is expected to grow from US$ 3,171.78 million in 2021 to US$ 6,628.39 million by 2028; it is estimated to grow at a CAGR of 11.1% from 2021 to 2028. The development of blockbuster therapies such as targeted gene therapies, specialty drugs, and precision medicine that help treat specific diseases and disorders has been a major focus in the healthcare sector for a long period. A few of these therapies are also being combined with medical devices to enhance the quality of drug delivery, dose, and patient monitoring or adherence, which is expected to add to the complexity of the related regulatory strategies and difficulties in their way to market. Thus, developments in emerging segments in healthcare sectors such as specialty therapies, orphan drugs, and personalized medicines are expected to offer significant growth opportunities to healthcare regulatory affairs outsourcing market players during the forecast period.
Key Market Segments
The North America healthcare regulatory affairs outsourcing market has been segmented based on service type, end user, and country. On the basis of service type, the North America healthcare regulatory affairs outsourcing market is segmented into medical & scientific writing, pharmacovigilance, data management services, life cycle management services, eCTD and e-Submissions, regulatory and scientific strategy development, chemistry manufacturing and controls (CMC) services, regulatory labelling, and regulatory artwork services. The medical & scientific writing segment dominated the market in 2020 and pharmacovigilance segment is expected to be the fastest growing during the forecast period. Based on end user, the market is segmented into pharmaceutical companies, biotechnology companies, and medical devices companies. The pharmaceutical companies segment dominated the market in 2020 and is expected to be the fastest growing during the forecast period. Likewise, the medical devices companies segmented is categorized into medical device materials & biomaterials, medical device, biomarkers and in vitro diagnostics (IVD), medical device software (SaMD), medical device electromechanics, medical device substance-based, and medical device of combination product.
Major Sources and Companies Listed
A few major primary and secondary sources referred to for preparing this report on healthcare regulatory affairs outsourcing market in North America are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Arriello Ireland Ltd., IQVIA Inc., PAREXEL INTERNATIONAL CORPORATION, PHARMALEX GMBH, ProductLife Group, ProPharma Group, and Voisin Consulting Life Sciences (VCLS) are among others.
Reasons to buy report
North America Healthcare Regulatory Affairs Outsourcing Market Segmentation
North America Healthcare Regulatory Affairs Outsourcing Market –By
Service Type
North America Healthcare Regulatory Affairs Outsourcing Market –By End User
North America Healthcare Regulatory Affairs Outsourcing Market -By Country
North America Healthcare Regulatory Affairs Outsourcing Market -
Company Profiles
The North America Healthcare Regulatory Affairs Outsourcing Market is valued at US$ 3,171.78 Million in 2021, it is projected to reach US$ 6,628.39 Million by 2028.
As per our report North America Healthcare Regulatory Affairs Outsourcing Market, the market size is valued at US$ 3,171.78 Million in 2021, projecting it to reach US$ 6,628.39 Million by 2028. This translates to a CAGR of approximately 11.1% during the forecast period.
The North America Healthcare Regulatory Affairs Outsourcing Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Healthcare Regulatory Affairs Outsourcing Market report:
The North America Healthcare Regulatory Affairs Outsourcing Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Healthcare Regulatory Affairs Outsourcing Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Healthcare Regulatory Affairs Outsourcing Market value chain can benefit from the information contained in a comprehensive market report.