North America ePRO, ePatient Diaries, and eCOA Market
No. of Pages: 131 | Report Code: TIPRE00022821 | Category: Technology, Media and Telecommunications
No. of Pages: 131 | Report Code: TIPRE00022821 | Category: Technology, Media and Telecommunications
The usage of electronic Clinical Outcome Assessment (eCOA) is a push towards adapting to the ‘new normal’ as it is a method to gather patient data electronically through the use of technology such as smart home devices, handheld monitors, wearables, e-diaries, tablets and web servers to allow the stakeholders in the trials (patients, healthy volunteers, investigators and caregivers) to report outcomes directly and digitally. Although historically COA was only related to the evaluation of Patient-Related Outcomes (PRO), the FDA has now broadened the definition to include PerfO, ClinRO and ObsRO along with PRO. In simple terms, when the above parameters are reported electronically, they fit under the eCOA spectrum.
eCOA/ePRO platform has substantial benefits for sponsors and CROs, as it reduces administrative burden, mitigates cost, and speeds trials. Such a system shows strong results with fewer errors and discrepancies, improved data quality, clearer signals, and standardized, accurate studies. The increasing adoption of EHR, and government regulations mandating maintain health records is driving the growth of the market.
Thus, a growing demand for clinical trials is expected to create a significant demand for ePRO, ePatient Diaries, and eCOA in the coming years, which is further anticipated to drive the ePRO, ePatient Diaries, and eCOA market.
The vaccine development for COVID-19 has a positive impact on developing new products in the market to handle research activities remotely. For instance, In April 2020, ArisGlobal has launched LifeSphere CTMS10. This modern, end-to-end solution makes the entire clinical trial management process more accessible and transparent for companies of all sizes. Furthermore, In March 2021, Signant Health has launched an eCOA solution for oncology trials, intending to accelerate research start-up and lower study costs, and ensuring accuracy and efficiency of results data collection among trial participants. These factors had a potential impact on the North America ePRO, e-Patient Diaries, and eCOA market.

Strategic insights for the North America ePRO, ePatient Diaries, and eCOA provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
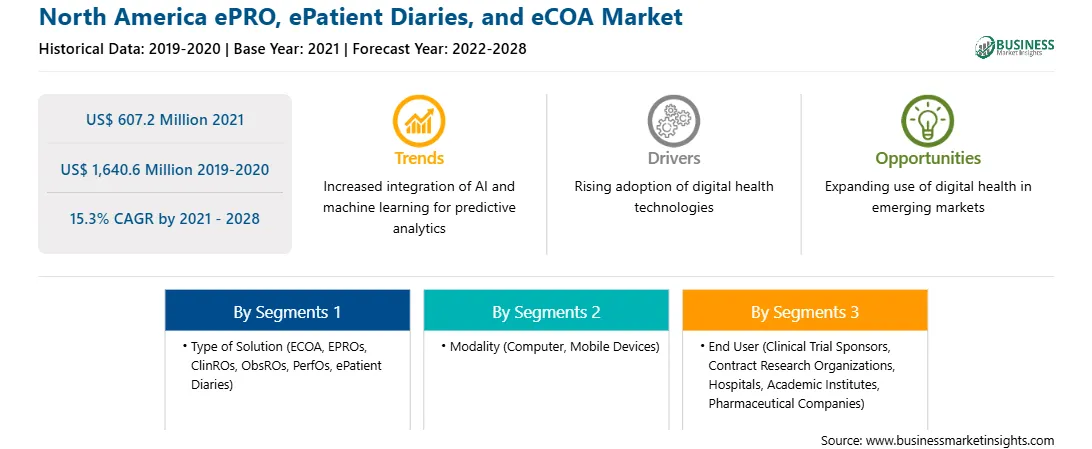
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 607.2 Million |
| Market Size by 2028 | US$ 1,640.6 Million |
| Global CAGR (2021 - 2028) | 15.3% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type of Solution
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America ePRO, ePatient Diaries, and eCOA refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The ePRO, ePatient Diaries, and eCOA market in North America is expected to grow from US$ 607.2 million in 2021 to US$ 1,640.6 million by 2028; it is estimated to grow at a CAGR of 15.3% from 2021 to 2028. With an increase in clinical trials and the need to be able to easily centralize data capture, as well as the need to drive down the overall cost of bringing a new drug to market, the adoption of ePRO, e-Patient Diaries, and eCOA platforms will be on rise as capturing data using eCOA solutions not only improves the quality of data, but also complements the data collection process across the study, leading to reduced risk and providing significant value. Additionally, majority of clinical trial stakeholders including sponsors, CROs and regulators consider eCOA as the most effective way to capture high-quality and more reliable data than traditional, paper-based methods.
In terms of type of solution, the e-clinical outcome assessments segment accounted for the largest share of the North America ePRO, ePatient Diaries, and eCOA market in 2020. In terms of modality, the mobile devices segment held a larger market share of the ePRO, ePatient Diaries, and eCOA market in 2020. In terms of end user, the cinical trial sponsors segment held a larger market share of the ePRO, ePatient Diaries, and eCOA market in 2020.
A few major primary and secondary sources referred to for preparing this report on the EPRO, ePatient Diaries, and eCOA market in North America are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are ERT Clinical, ArisGlobal LLC, The Diary Pte. Ltd, ICON PLC, PAREXEL INTERNATIONAL CORPORATION, Anju Software, Inc., Kayentis, Bracket Global LLC, Dassault Systèmes SE, CRF Health, and eClinical Solutions.
By Type of Solution
By Modality
By End User
By Country
The North America ePRO, ePatient Diaries, and eCOA Market is valued at US$ 607.2 Million in 2021, it is projected to reach US$ 1,640.6 Million by 2028.
As per our report North America ePRO, ePatient Diaries, and eCOA Market, the market size is valued at US$ 607.2 Million in 2021, projecting it to reach US$ 1,640.6 Million by 2028. This translates to a CAGR of approximately 15.3% during the forecast period.
The North America ePRO, ePatient Diaries, and eCOA Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America ePRO, ePatient Diaries, and eCOA Market report:
The North America ePRO, ePatient Diaries, and eCOA Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America ePRO, ePatient Diaries, and eCOA Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America ePRO, ePatient Diaries, and eCOA Market value chain can benefit from the information contained in a comprehensive market report.