The application of artificial intelligence (AI) in gastrointestinal (GI) endoscopy is drawing a great amount of attention because it has the potential to improve the quality of endoscopy at all levels, compensating for human errors and limitations by bringing more accuracy, consistency, and higher speed. It will make a breakthrough and a big revolution in the development of GI endoscopy. AI has the advantage of limiting inter-operator variability. It can compensate for the limited experience of novice endoscopists and the errors of even the most experienced endoscopists. Over the past four decades, the incidence of esophageal adenocarcinoma (EAC) has risen rapidly due to the increasingly prevalent excess body weight. AI assistance shows promising results in terms of improving the detection and diagnosis of esophageal adenocarcinoma (EAC), thus, reducing the mortality and morbidity related to this type of malignancy with a poor prognosis when diagnosed at an advanced stage.
The Canadian Association of Gastroenterology (CAG) has formed a special interest group (SIG) in AI to further develop and promote the use of AI. This CAG AI SIG core group comprises six gastroenterologists from five Canadian institutions across three provinces. They have started evaluating AI technologies using cohort studies and randomized controlled trials. They are in the process of establishing video and data biobanks to accrue raw data from which additional novel AI solutions can be created. Further activities of group members include developing and implementing AI curricula since the next generation of gastroenterologists needs to be trained to develop and implement AI solutions at institutions across Canada. The CAG AI SIG has an open model inviting new members, and AI researchers to maximize this novel technology's potential in improving endoscopy quality and patient outcomes.
Recently, a few AI-driven endoscopy products were approved in North American region. For instance, in November 2021, Medtronic Canada ULC, a subsidiary of Medtronic plc, announced that it had received a Health Canada license for the GI Genius intelligent endoscopy module. GI Genius is a computer-aided detection (CADe) system that uses artificial intelligence (AI) to highlight regions of the colon suspected to have visual characteristics consistent with different types of mucosal abnormalities. Hence, the use of artificial intelligence (AI) in gastroenterology is likely to propel the growth of endoscopy procedures in near future.
Ibex Medical Analytics, a pioneer in AI-powered cancer diagnostics, developed the world’s first artificial intelligence (AI) platform to detect cancer. Its AI algorithms analyze images of biopsies and can pinpoint their location and grade the tumor. These results help pathologists diagnose gastric cancer accurately. In June 2021, the U.S. Food and Drug Administration (FDA) granted Breakthrough Device Designation to Ibex’s Galen platform, which will expedite the clinical review and regulatory approval of its platform. Galen Gastric is an integrated diagnostics and quality control solution that supports pathologists in the detection of gastric cancer, dysplasia, H. pylori, and other important clinical findings.
In November 2019, AI Medical Service Inc., one of the world's first real-time endoscopic AI developers, secured Breakthrough Device Designation by the US FDA for its AI programs that analyze endoscopy images for potential diagnosis of gastric cancer.
Thus, such development of AI-based endoscopes for cancer diagnostics are likely to introduce new trends in the endoscopy procedure market during the forecast period.
The North America endoscopy procedure market is segmented into US, Canada, and Mexico. US is the largest market for endoscopy procedure followed by Canada and Mexico. Growing preference for minimally invasive surgeries and increasing prevalence of chronic diseases in the US and North America are likely to drive the endoscopy procedure market in the forecasted period. In addition, the technological advancement by market players leading to enhanced applications is likely to foster the growth of the market in the forecasted period.
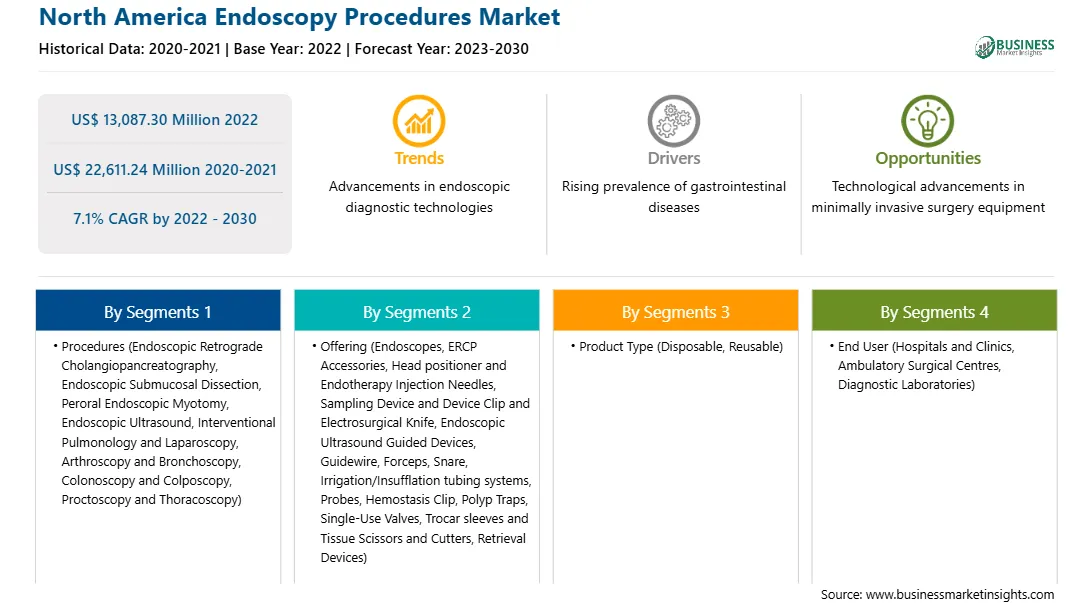
The North America endoscopy procedures market is segmented into procedure, offering, product type, and end user.
Based on procedure, the North America endoscopy procedures market is segmented into endoscopic retrograde cholangiopancreatography (ERCP), endoscopic submucosal dissection (ESD), peroral endoscopic myotomy (POEM), endoscopic ultrasound (EUS), interventional pulmonology and laparoscopy, arthroscopy and bronchoscopy, colonoscopy and colposcopy, proctoscopy and thoracoscopy, and others. The others segment held the largest share of the North America endoscopy procedures market in 2022.
Based on offering, the North America endoscopy procedures market is segmented endoscopes, ERCP accessories, visualization system, head positioner and endotherapy injection needles, sampling device and device clip and electrosurgical knife, endoscopic ultrasound guided devices, guidewire, forceps, snare, irrigation/insufflation tubing systems, probes, hemostats clip, polyps’ traps, single-use valves, trocar sleeves and tissue scissors and cutters, retrieval devices, and others. The endoscopes segment held the largest share of the North America endoscopy procedures market in 2022.
Based on product type, the North America endoscopy procedures market is bifurcated into disposable and reusable. The reusable segment held a larger share of the North America endoscopy procedures market in 2022.
Based on end user, the North America endoscopy procedures market is segmented into hospitals and clinics, ambulatory surgical centres, diagnostic laboratories, and others. The hospitals and clinics segment held the largest share of the North America endoscopy procedures market in 2022.
Based on country, the North America endoscopy procedures market is segmented into the US, Canada, and Mexico. The US dominated the North America endoscopy procedures market in 2022.
Stryker Corp, Merit, Medical Systems Inc, Smith & Nephew Plc, Arthrex Inc, Steris Plc, Conmed Corp, Olympus Corp, Boston Scientific Corp, and Cook Medical LLC are some of the leading companies operating in the North America endoscopy procedures market.
Strategic insights for the North America Endoscopy Procedures provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 13,087.30 Million |
| Market Size by 2030 | US$ 22,611.24 Million |
| Global CAGR (2022 - 2030) | 7.1% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Procedures
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America Endoscopy Procedures refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The North America Endoscopy Procedures Market is valued at US$ 13,087.30 Million in 2022, it is projected to reach US$ 22,611.24 Million by 2030.
As per our report North America Endoscopy Procedures Market, the market size is valued at US$ 13,087.30 Million in 2022, projecting it to reach US$ 22,611.24 Million by 2030. This translates to a CAGR of approximately 7.1% during the forecast period.
The North America Endoscopy Procedures Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Endoscopy Procedures Market report:
The North America Endoscopy Procedures Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Endoscopy Procedures Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Endoscopy Procedures Market value chain can benefit from the information contained in a comprehensive market report.