Major players in the colorectal cancer diagnostic market manufacture a wide range of devices that help reduce the burden of colorectal cancer and other associated indications such as colon polyps, Crohn’s disease, colitis, and irritable bowel syndrome. In July 2022, US Digestive Health (USDH), a network of top-rated gastrointestinal (GI) practices, announced the commercialization of AI-assisted colonoscopy screenings with the country’s largest installation of Genious Intelligent GI endoscopy modules. These modules are expected to help physicians identify hard-to-detect and potentially cancerous polyps in real time. With the launch of this device, patients throughout southeastern, southwestern, and central Pennsylvania can now access AI-assisted colonoscopy with enhanced capabilities. In September 2020, Olympus Corporation announced the launch of ENDO-AID, a cutting-edge platform powered by artificial intelligence (AI). The platform includes ENDO-AID CADe application (app), a computer-aided endoscopic method for the detection of different conditions of the colon. This new AI platform enables the real-time display of automatically detected suspicious lesions and works in combination with its EVIS X1. Thus, the frequent developments and new product launches drive the North America colorectal cancer diagnostics market growth.
The North America colorectal cancer diagnostics market is segmented into the US, Canada, and Mexico. According to the American Society of Clinical Oncology, colorectal cancer is the third most common cancer diagnosed in men and women in the US. In 2023, an estimated 153,020 adults in the US will be diagnosed with colorectal cancer. These numbers include 106,970 new cases of colon cancer (54,420 men and 52,550 women) and 46,050 new cases of rectal cancer (27,440 men and 18,610 women). Further, ~1,880,725 people were diagnosed with colorectal cancer in 2020. Although older adults are more prone to colorectal cancer, the incidence of cancer is increasing among younger adults. The lifetime risk of getting affected by colorectal cancer is ~1 in 22 for men and ~1 in 24 for women. The American Society of Clinical Oncology (ASCO) estimated that ~52,550 deaths from colorectal cancer disease will occur in the US in 2023. In 2022, more than 600,000 surgeries were performed annually in the US to treat colon diseases. According to the American Cancer Society, the five-year survival rate for localized colon cancer is 91% and for localized rectal cancer is 90%. Overall, colorectal cancer mortality rates have been declined with better screening and treatment over the past 20 years. Moreover, screening reduces CRC mortality by decreasing incidences of CRC and increasing survival rates. The 2018 American Cancer Society CRC screening guideline recommends that adults aged 45 years and above must undergo regular screening with a high-sensitivity stool-based test or visual examination, depending on patient preference and test availability. In May 2021, the US Preventive Services Task Force changed its colorectal cancer screening recommendation. The age at which adults were at average risk of being diagnosed with colorectal cancer and recommended to begin screening was lowered from 50 to 45. Also, the government of the country supports research on colorectal cancer. The researchers worked to develop an advanced technique that can be used to prevent, detect, and treat colorectal cancer. The Colorectal Cancer Alliance is the world's largest nonprofit colorectal cancer patient advocacy organization dedicated to funding colorectal cancer research. In 2020, an additional US$ 1.1 million in funding was made to support individual scientists through research grants. Thus, owing to abovementioned factors, the North America colorectal cancer diagnostics market is likely to propel during the forecast period.
Strategic insights for the North America Colorectal Cancer Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the North America Colorectal Cancer Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.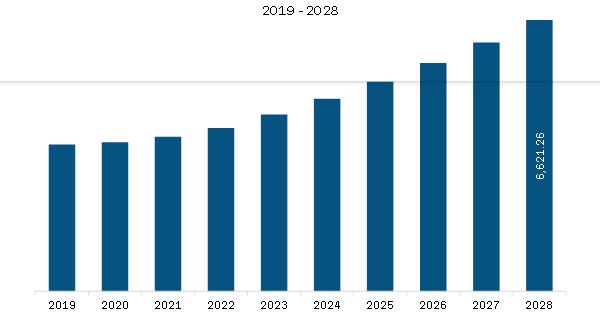
North America Colorectal Cancer Diagnostics Strategic Insights
North America Colorectal Cancer Diagnostics Report Scope
Report Attribute
Details
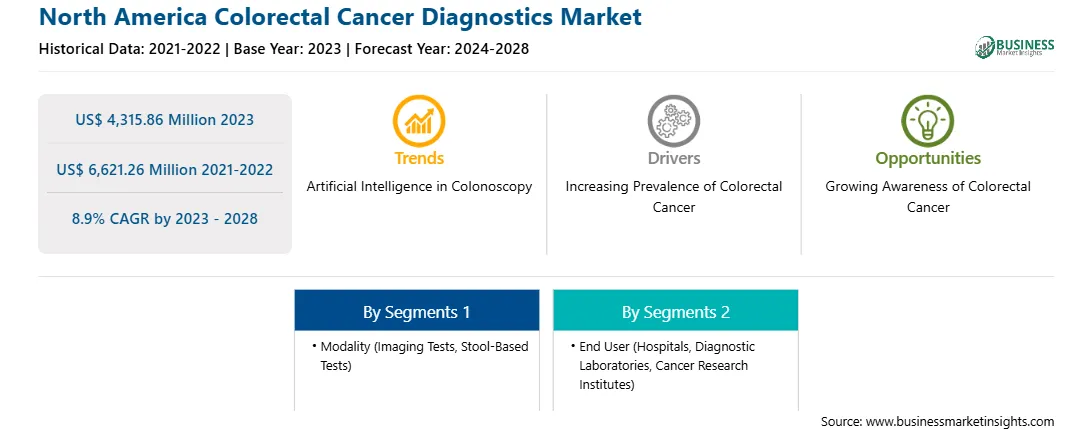
Market size in 2023
US$ 4,315.86 Million
Market Size by 2028
US$ 6,621.26 Million
Global CAGR (2023 - 2028)
8.9%
Historical Data
2021-2022
Forecast period
2024-2028
Segments Covered
By Modality
By End User
Regions and Countries Covered
North America
Market leaders and key company profiles
North America Colorectal Cancer Diagnostics Regional Insights
North America Colorectal Cancer Diagnostics Market Segmentation
The North America colorectal cancer diagnostics market is segmented into modality, end user, and country.
Based on modality, the North America colorectal cancer diagnostics market is bifurcated into imaging tests and stool-based tests. In 2023, the imaging tests segment held a larger share of the North America colorectal cancer diagnostics market. The market for the imaging tests segment is further segmented into colonoscopy, CT colonography, flexible sigmoidoscopy, capsule endoscopy, and others. The market for the stool based tests segment is subsegmented into faecal immunochemical test (fit), guaiac-based faecal occult blood test (gFOBT), and stool DNA test.
Based on end user, the North America colorectal cancer diagnostics market is segmented into hospitals, diagnostic laboratories, cancer research institutes, and others. In 2023, the hospitals segment held the largest share of the North America colorectal cancer diagnostics market.
Based on country, the North America colorectal cancer diagnostics market is segmented into the US, Canada, and Mexico. In 2023, the US accounted for the largest share of the North America colorectal cancer diagnostics market.
Medtronic Plc; Illumina Inc; Clinical Genomics Technologies Pty Ltd; EDP Biotech Corp; Epigenomics AG; F. Hoffmann-La Roche Ltd; Quest Diagnostics Inc; Siemens Healthineers AG; Bruker Corp; and Eiken Chemical Co., Ltd. are among the leading companies operating in the North America colorectal cancer diagnostics market.
The North America Colorectal Cancer Diagnostics Market is valued at US$ 4,315.86 Million in 2023, it is projected to reach US$ 6,621.26 Million by 2028.
As per our report North America Colorectal Cancer Diagnostics Market, the market size is valued at US$ 4,315.86 Million in 2023, projecting it to reach US$ 6,621.26 Million by 2028. This translates to a CAGR of approximately 8.9% during the forecast period.
The North America Colorectal Cancer Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Colorectal Cancer Diagnostics Market report:
The North America Colorectal Cancer Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Colorectal Cancer Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Colorectal Cancer Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.