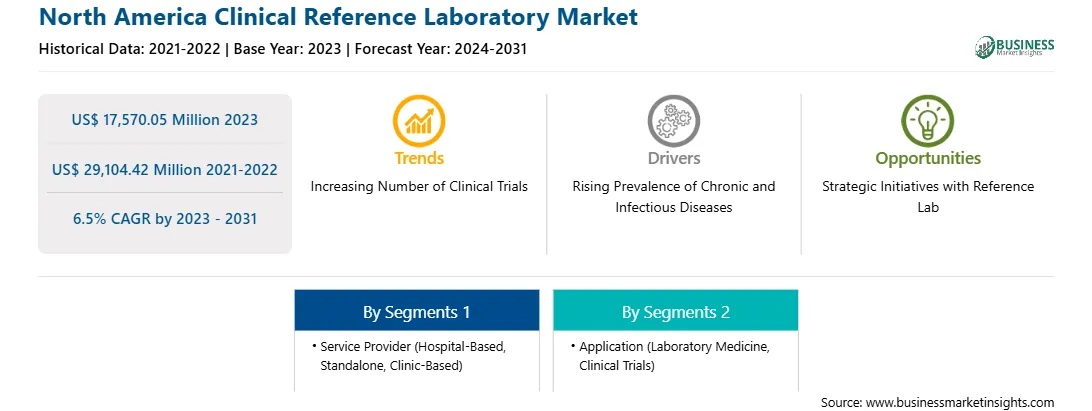
The North America clinical reference laboratory market was valued at US$ 17,570.05 million in 2023 and is expected to reach US$ 29,104.42 million by 2031; it is estimated to register a CAGR of 6.5% from 2023 to 2031. Rising Prevalence of Chronic and Infectious Diseases Fuels North America Clinical Reference Laboratory Market
The increasing prevalence of chronic diseases and the emergence and outbreak of various infectious diseases have fueled the demand for novel treatment options and newer therapeutic categories and created the need for better treatment options in existing therapeutic categories. According to the WHO data released in September 2023, noncommunicable diseases (NCDs), including heart disease, cancer, stroke, diabetes, and chronic lung disease, kill 41 million people each year. According to the United Health Foundation, more than 29 million US adults reported having three or more chronic conditions in 2022. Accurate diagnostics are critical for identifying disease biomarkers and detecting organisms, particularly in cancer and tuberculosis. Clinical reference laboratories play an important role in modern healthcare. The reference laboratories provide services for the quick diagnosis of infectious diseases that require specialized laboratory testing equipment and expertise, such as swine flu, hepatitis A and B. These laboratories also offer low-cost diagnostic testing for common chronic conditions such as cancer, diabetes, and cardiovascular disease. During the COVID-19 pandemic, it became evident that medical reference labs are critical for rapid testing, establishing novel techniques, generating and analyzing data, and serving as knowledge hubs. Reference labs are more than just high-volume testing facilities; they are the hubs of expertise and data. Thus, the rising prevalence of infectious and chronic diseases is driving the growth of the clinical reference laboratory market.North America Clinical Reference Laboratory Market Overview
The US is one of the leading clinical research destinations. Nearly half of the total clinical trials are performed in the US. Additionally, most pharmaceutical research companies choose to perform clinical trials in the US owing to fast approval timelines, a favorable regulatory framework, and established medical infrastructure. Moreover, data generated for clinical trials in the US is accepted globally. According to the World Health Organization (WHO) report in 2021, the US registered the highest number of clinical trials (157,618).
The following table shows the number of clinical trials registered in the US, the number of total patients recruited in them, and the percentage share of the US for the given parameters globally.
2023 | Registered Clinical Trial Studies | Patient Recruited in Studies |
US | 139,632 (31% of the global studies) | 20,680 (32% of the worldwide headcount) |
Source: ClinicalTrial.gov report
The expanding access of companies through mergers and acquisitions further boosts the growth of the clinical reference laboratory market in the US. For instance, in December 2020, Clinical Reference Laboratory (CRL)-a privately held clinical testing laboratory in the US-acquired Confirm BioSciences, a leading provider of comprehensive screening tools and solutions. Confirm will continue as a subsidiary under its current name. The acquisition expands Confirm's access to lab testing services and R&D capabilities. NCEZID laboratories, Mayo Clinic Laboratories, and CRL are among the leading reference laboratories for clinical trials. The growing number of clinical trials in the US favors the growth of the clinical reference laboratory market in the country.
Strategic insights for the North America Clinical Reference Laboratory provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the North America Clinical Reference Laboratory refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.North America Clinical Reference Laboratory Strategic Insights
North America Clinical Reference Laboratory Report Scope
Report Attribute
Details
Market size in 2023
US$ 17,570.05 Million
Market Size by 2031
US$ 29,104.42 Million
Global CAGR (2023 - 2031)
6.5%
Historical Data
2021-2022
Forecast period
2024-2031
Segments Covered
By Service Provider
By Application
Regions and Countries Covered
North America
Market leaders and key company profiles
North America Clinical Reference Laboratory Regional Insights
North America Clinical Reference Laboratory Market Segmentation
The North America clinical reference laboratory market is categorized into service provider, application, and country.
Based on service provider, the North America clinical reference laboratory market is segmented into hospital-based, standalone, and clinic-based. The hospital-based segment held the largest market share in 2023.
In terms of application, the North America clinical reference laboratory market is categorized into laboratory medicine, clinical trials, and others. The laboratory medicine segment held the largest market share in 2023.
By country, the North America clinical reference laboratory market is segmented into the US, Canada, and Mexico. The US dominated the North America clinical reference laboratory market share in 2023.
Sonic Healthcare Ltd, Laboratory Corp of America Holdings, Quest Diagnostics Inc, ARUP Laboratories Inc, Clinical Reference Laboratory Inc, SYNLAB International GmbH, Myraid Genetics Inc, Boca Biolistics LLC, and TOPLAB are some of the leading companies operating in the North America clinical reference laboratory market.
The North America Clinical Reference Laboratory Market is valued at US$ 17,570.05 Million in 2023, it is projected to reach US$ 29,104.42 Million by 2031.
As per our report North America Clinical Reference Laboratory Market, the market size is valued at US$ 17,570.05 Million in 2023, projecting it to reach US$ 29,104.42 Million by 2031. This translates to a CAGR of approximately 6.5% during the forecast period.
The North America Clinical Reference Laboratory Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Clinical Reference Laboratory Market report:
The North America Clinical Reference Laboratory Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Clinical Reference Laboratory Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Clinical Reference Laboratory Market value chain can benefit from the information contained in a comprehensive market report.