Health systems in this region are witnessing a substantial increase in the prevalence of cancer. According to the International Agency for Research on Cancer (IARC), cancer burden in North America has increased to 19.3 million cases and 9.96 million cancer deaths as of 2020. A study published by the National Cancer Institute estimated the cancer incidence in the US at 1.9 million in 2021. ~0.6 million people were anticipated to die in 2021 in the US due to cancer. Thus, the increased prevalence of cancer across North America is propelling the demand for circulating tumor cell (CTC) diagnostics. The analysis and detection of circulating tumor cells assist in early patient diagnosis, and prognosis helps determine accurate treatment for the patient. The CTC diagnostics can be used to detect various types of cancers, including prostate cancer, breast cancer, colorectal cancer, kidney cancer, lymphomas, lung cancer, and melanoma. There has been a high demand for CTC diagnostics in recent years due to its reliability and accuracy in identifying the risk of the disease and monitoring its treatments.
The impact of the COVID-19 pandemic on cancer patients, cancer surveillance capacities, and the whole American health system has been notable. Reduced resources and limited healthcare capacity made cancer detection and treatment more difficult, resulting in lower incidence, higher mortality, and lower survival in the future. Because of a compromised immune system caused by cancer and/or its treatment (e.g., surgery and chemotherapy), people with active cancer are more susceptible to infectious pathogens. Thus, the risk of COVID-19 severity and mortality may be more common in cancer patients. Males; people of age 60 and above; people with a history of smoking; populations suffering from obesity, hypertension, cardiovascular disease, and diabetes have all been consistently related to an elevated risk of severe disease and/or death. Findings relating to prognosis for cancer-associated variables have proven conflicting.
With new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the circulating tumor cell (CTC) diagnostics market in North America at a good CAGR during the forecast period.
North America Circulating Tumor Cell (CTC) Diagnostics Market Segmentation
Strategic insights for the North America Circulating Tumor Cell (CTC) Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
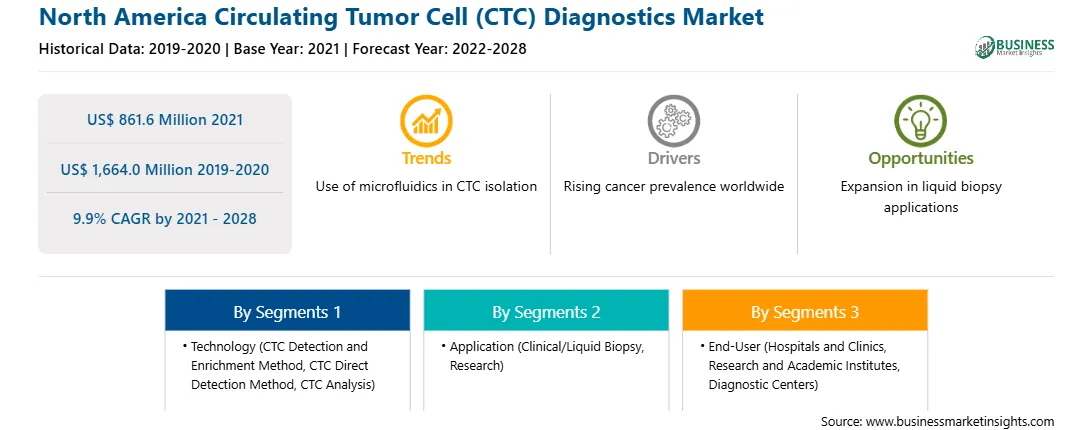
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 861.6 Million |
| Market Size by 2028 | US$ 1,664.0 Million |
| Global CAGR (2021 - 2028) | 9.9% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Technology
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America Circulating Tumor Cell (CTC) Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
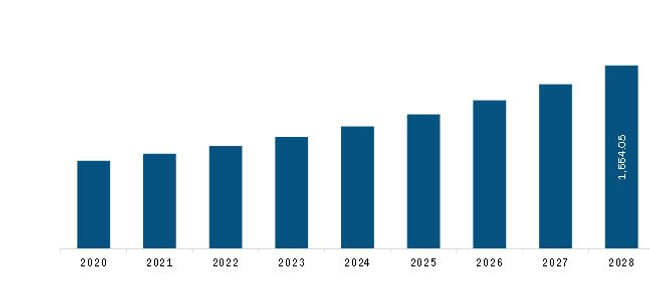
The North America Circulating Tumor Cell (CTC) Diagnostics Market is valued at US$ 861.6 Million in 2021, it is projected to reach US$ 1,664.0 Million by 2028.
As per our report North America Circulating Tumor Cell (CTC) Diagnostics Market, the market size is valued at US$ 861.6 Million in 2021, projecting it to reach US$ 1,664.0 Million by 2028. This translates to a CAGR of approximately 9.9% during the forecast period.
The North America Circulating Tumor Cell (CTC) Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Circulating Tumor Cell (CTC) Diagnostics Market report:
The North America Circulating Tumor Cell (CTC) Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Circulating Tumor Cell (CTC) Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Circulating Tumor Cell (CTC) Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.