In August 2020, the World Health Organization (WHO) devised a strategy for the elimination of cervical cancer. Per this strategy, all countries across the region must focus on reaching and maintaining an incidence rate of less than 4 per 100,000 women to eliminate cervical cancer. To attain such low incidence rates, countries should focus on cervical cancer screening, treatment, and vaccination. Medicare, a popular government insurance program, provides coverage for PAP tests, pelvic exams, and clinical breast examinations performed every two years for cervical cancer screening.
Unitaid, a global health agency that works to bring about innovative solutions to prevent, diagnose, and treat major diseases in low- and middle-income countries, has come up with a new initiative for cervical cancer prevention. Its efforts are focused on expanding access to critical tools and services to screen for the early signs of cervical cancer, followed by the treatment of positive cases. Unitaid is providing support for the Clinton Health Access Initiative (CHAI) and the Scale Up Cervical Cancer Elimination with Secondary Prevention Strategy (SUCCESS) Project, which are being carried out in partnership with Expertise France, Jhpiego, and the Union for International Cancer Control (UICC). Unitaid is collaborating with these partners and governments of 14 low- and middle-income countries to develop an affordable and highly effective package of tools, which can help the World Health Organization achieve its cervical cancer elimination targets. The Unitaid has invested ~US$ 70 million in innovative tools to screen women living in low-resource environments for precancer conditions and treat them. In 14 countries, Unitaid is striving to overcome access barriers and laying the groundwork for national cervical cancer elimination efforts, demonstrating effective models of prevention across low- and middle-income countries.
Such initiatives by various healthcare organizations and governments for preventing cervical cancer boost the growth of the CIN & HR-HPV treatment market.
The North America CIN & HR-HPV treatment market is segmented into the US, Canada, and Mexico. The US held the largest share of the market in 2022. The rising number of research and development activities, growing awareness of cervical cancer, the significant presence of healthcare giants, and initiatives by government authorities and NGOs to promote cervical cancer screening are the factors contributing to the growth of the CIN & HR-HPV treatment market.
Strategic insights for the North America CIN & HR-HPV Treatment provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
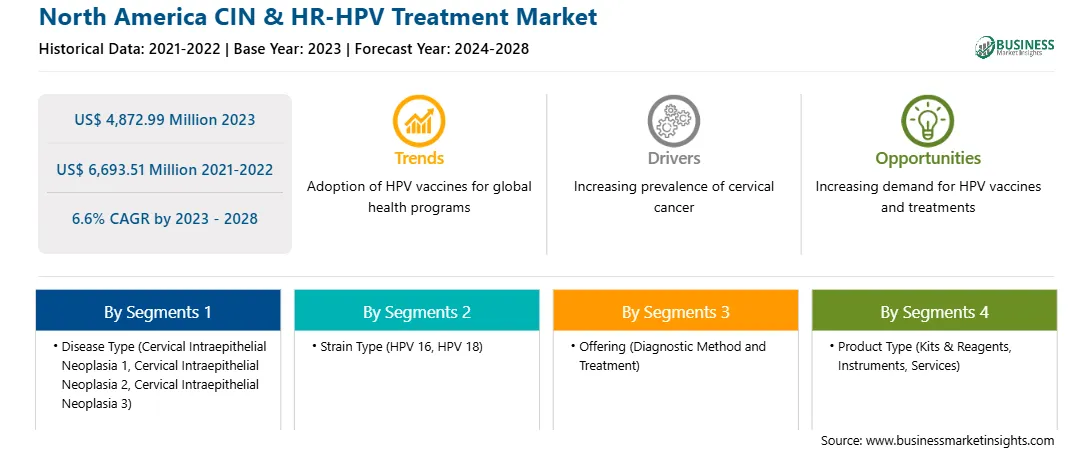
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 4,872.99 Million |
| Market Size by 2028 | US$ 6,693.51 Million |
| Global CAGR (2023 - 2028) | 6.6% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2028 |
| Segments Covered |
By Disease Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America CIN & HR-HPV Treatment refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The North America CIN & HR-HPV treatment market is segmented into disease type, strain type, offering, product type, end user, and country. Based on disease type, the market is subsegmented into cervical intraepithelial neoplasia 1 (CIN 1), cervical intraepithelial neoplasia 2 (CIN 2), and cervical intraepithelial neoplasia 3 (CIN 3). The cervical intraepithelial neoplasia 3 (CIN 3) segment registered the largest market share in 2023.
Based on strain type, the market is segmented into HPV 16, HPV 18, and others. The HPV 16 segment held a largest market share in 2023.
Based on offering, the market is bifurcated into diagnostic methods and treatments. The treatments segment held the largest market share in 2023. Further, diagnostic methods are segmented into pap smear, HPV testing, colposcopy, and biopsy. Further, treatments are bifurcated into excision surgery and ablation techniques.
Based on product type, the market is segmented into kits & reagents, instruments, and services. The services segment held a largest market share in 2023.
Based on end user, the market is segmented into hospitals & clinics, diagnostic laboratories, specialized clinical laboratories, and others. The hospitals & clinics segment held a largest market share in 2023.
Based on country, the market is segmented into the US, Canada, Mexico. The US dominated the market share in 2023.
Fujirebio Europe NV; Qiagen NV; Abbott Laboratories; Cepheid; F. Hoffmann-LA Roche Ltd; INOVIO Pharmaceuticals Inc; Bioneer Corp; Antiva Biosciences Inc; and Thermo Fisher Scientific Inc are the leading companies operating in the CIN & HR-HPV treatment market in the region.
The North America CIN & HR-HPV Treatment Market is valued at US$ 4,872.99 Million in 2023, it is projected to reach US$ 6,693.51 Million by 2028.
As per our report North America CIN & HR-HPV Treatment Market, the market size is valued at US$ 4,872.99 Million in 2023, projecting it to reach US$ 6,693.51 Million by 2028. This translates to a CAGR of approximately 6.6% during the forecast period.
The North America CIN & HR-HPV Treatment Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America CIN & HR-HPV Treatment Market report:
The North America CIN & HR-HPV Treatment Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America CIN & HR-HPV Treatment Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America CIN & HR-HPV Treatment Market value chain can benefit from the information contained in a comprehensive market report.