Skin cancer means abnormal growth of skin cells. It develops in the areas exposed to the sun. Ultraviolet radiation changes the genetic material (DNA) in cells and is the leading cause of skin cancer. Skin cancer is the most common form of cancer. There are two main categories of skin cancer such as melanoma and non-melanoma. Various imaging solutions such as CT scans, MRIs, PET scans, bone scans, and chest X-rays are used to diagnose skin cancer. The skin cancer diagnostics market also includes various diagnostics and therapies related to skin cancer, such as chemotherapy, targeted therapy, immunotherapy, radiotherapy, freezing treatment, and scraping therapy that helps cure skin cancer.
The North America and Europe skin cancer diagnostics market is segmented on the bases of type, screening type, and geography. By geography, the market is broadly segmented into North America and Europe. The report offers insights and in-depth analysis of the market, emphasizing on parameters such as market trends, technological advancements, and market dynamics along with the analysis of competitive landscape of world’s leading market players.
According to the World Health Organization (WHO), in 2020, cancer accounted for 10 million deaths worldwide. Breast, lung, colon and rectum, prostate, skin, and stomach cancer were the commonly seen types in the world in that year. According to the WHO data, ~1.20 million of the new cases reported in 2020 were skin cancer cases. Skin cancer is by far the most common of all cancer types. Melanoma and non-melanoma are the two types of skin cancer. Although melanoma accounts for ~1% of total skin cancer cases, it causes maximum skin cancer deaths. According to the Skin Cancer Foundation, during 2012–2022, annual diagnosis for invasive melanoma cases increased by 31%. As per GLOBOCAN 2020, worldwide 19.3 million new cancer cases, excluding 18.1 million nonmelanoma skin cancer cases, were registered in 2020. Several countries have adopted national programs for cancer awareness and early cancer diagnosis. Furthermore, increased exposure of people to ultraviolet rays leads to genetic mutations, which may cause skin cancer in the long term. The growing awareness about such aspects drives the skin cancer diagnostics market growth.
The need for the timely diagnosis of skin cancer boosts the demand for better and advanced diagnosis solutions. In response to such prominent demand, various companies focus on offering better products for the diagnosis of skin cancer. In January 2020, 3Derm Systems, Inc. received two FDA Breakthrough Device designations for its 3DermSpot. 3DermSpot is an AI-based algorithm that autonomously detects melanoma, squamous cell carcinoma, and basal cell carcinoma using standardized skin images saved in it. In May 2021, this device was awarded a breakthrough device designation by the FDA. Presently, the company is awaiting to receive FDA approval for its commercial launch in the US.
In May 2021, SciBase Holding AB launched its Non-Melanoma Skin Cancer (NMSC) clinical application (app) and MDR certification process. The NMSC application is designed to support the company’s Nevisense technology platform. The Nevisense, which helps detect melanoma, is a CE-marked device available in Europe; moreover, it has received the TGA and FDA approvals for commercial use in the US, respectively. The company is looking forward to integrating the NMSC clinical application into its Nevisense 3.0 version. In December 2021, Foundation Medicine, Inc. received FDA approval for its FoundationOneCDx. The FoundationOneCDx is a next-generation sequencing-based in-vitro diagnostic device. It is FDA approved therapeutics in melanoma for present and future. It is enabled with the BRAF inhibitor monotherapies targeting the BRAFV600E mutations and BRAF/MEK inhibitor combination therapies targeting the BRAFV600E or V600K mutations. It is considered an important step toward enabling simplified decision-making for oncologists.
Such innovations by cancer diagnostics companies are driving the North America and Europe skin cancer diagnostics market growth.
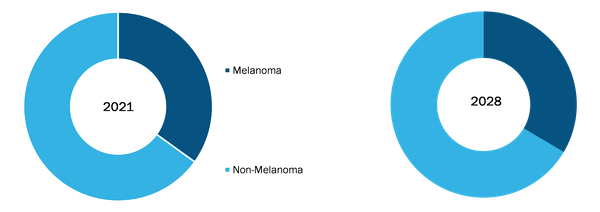
Based on type, the North America and Europe skin cancer diagnostics market is segmented into melanoma and non-melanoma. The non-melanoma segment held the largest share of the market in 2021. Moreover, the market for the non-melanoma segment is expected to grow at the highest CAGR during 2021–2028. Non-melanoma skin cancers are more frequent than melanoma skin cancer. These are completely curable through surgery and are rarely lethal, however, surgical treatment is painful and disfiguring. According to the Skin Cancer Foundation, it is stated that over 5, 400 people globally die of non-melanoma skin cancer every month. Various countries across the world are raising awareness with respect to non-melanoma skin cancer. For instance, in June 2019, The European Cancer Patient Coalition (ECPC) and the European Academy of Dermatology and Venereology (EADV) has launched the Global Non-Melanoma Skin Cancer (NMSC) Awareness Day to raise awareness of NMSC.
Based on screening type, the North America and Europe skin cancer diagnostics market is segmented into blood tests, dermatoscopy, imaging tests, lymph node biopsy, and skin biopsy. The skin biopsy segment held the largest market share in 2021, and it is further expected to be the largest shareholder in the market by 2028. Skin biopsy is diagnosis procedure that require a small sample of the skin. It is performed to determine skin cancer, infection or other skin conditions. These types of biopsies are performed for melanoma skin cancer. And in cases of non-melanoma, biopsy is often the only test done to diagnose the stage or the extension of the cancer as non-melanoma skin cancer rarely spreads. The increasing awareness and incidences of skin cancer are likely to drive the North America and Europe skin cancer diagnostics market during the forecast period.
Product launches, and mergers and acquisitions are the highly adopted strategies by the players operating in the North America and Europe skin cancer diagnostics market. A few of the recent key product developments are listed below:
In August 2020, Michelson Diagnostics Ltd. had announced the launch of VivoTools, an advanced software suite for the analysis of VivoSight Dx OCT images. VivoSight Dx isone of the leading Optical Coherence Tomography (OCT) skin imaging system which can see over 1 mm deep into the skin to produce images of skin structural elements, vascularity and pathology. The company have made a couple of significant advances to the image analysis software, resulting in the debut of a new brand, called VivoTools.
In December 2021, BioIQ, Inc. and DermTech, Inc. has collaborated to bring increased patient access to precision genomics. The DermTech Melanoma Test is now included in BioIQ's lineup of testing options for employee and member health programs.
In December 2021, SkylineDx had announced a 3-year strategic partnership with life sciences institute VIB for the evaluation and initiation of collaborative projects focused on molecular diagnostics. The collaboration aims to progress high potential research projects into in vitro diagnostic device (IVD) development trajectories to create commercially viable products linked to unmet medical needs for global markets.
The COVID-19 pandemic also has a substantial impact on diagnostics in the regions due to the closure of practices. The COVID-19 pandemic has radically changed clinical and surgical routines, profoundly impacting all aspects of healthcare and surgical practice, from the workforce and prioritization of procedures to the risk of viral disease and intraoperative transmission. According to a research article published in March 2021, universal healthcare claims in Ontario showed drastic drop in total skin biopsies. There was 15% drop in biopsies from the expected rate. There were huge backlogs in skin biopsies during the lockdown. The UK healthcare system has been severely affected by the COVID-19 pandemic. The UK healthcare system, including skin cancer departments, has been hit hard by the COVID-19 pandemic. Thus, the pandemic has negatively impacted the market for skin cancer diagnosis. However, the reopening of all medical services are expected to increase the demand for skin cancer diagnosis further leading to vital market growth.
The skin cancer diagnostics market is segmented on the basis of type, and screening type. Based on type, the market is segmented into melanoma and non-melanoma. By screening type, the skin cancer diagnostics market is segmented into skin biopsy, dermatoscopy, lymph node biopsy, imaging tests, and blood tests. By geography, the market is segmented into North America (the US, Canada, and Mexico) and Europe (France, Germany, the UK, Spain, Italy, and the Rest of Europe).
Strategic insights for the North America and Europe Skin Cancer Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
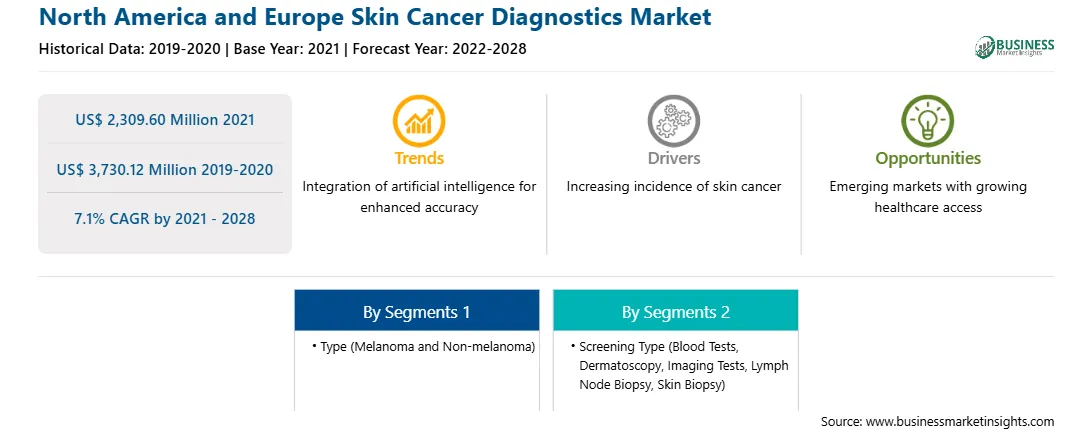
| Market size in 2021 | US$ 2,309.60 Million |
| Market Size by 2028 | US$ 3,730.12 Million |
| Global CAGR (2021 - 2028) | 7.1% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
The geographic scope of the North America and Europe Skin Cancer Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The North America and Europe Skin Cancer Diagnostics Market is valued at US$ 2,309.60 Million in 2021, it is projected to reach US$ 3,730.12 Million by 2028.
As per our report North America and Europe Skin Cancer Diagnostics Market, the market size is valued at US$ 2,309.60 Million in 2021, projecting it to reach US$ 3,730.12 Million by 2028. This translates to a CAGR of approximately 7.1% during the forecast period.
The North America and Europe Skin Cancer Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America and Europe Skin Cancer Diagnostics Market report:
The North America and Europe Skin Cancer Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America and Europe Skin Cancer Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America and Europe Skin Cancer Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.