New Product Launches and Approvals
The increasing prevalence of various chronic disorders, such as asthma, COPD, cystic fibrosis, and emphysema, among a large population has fueled new product developments, product launches, and approvals on a global level. Additionally, major market players are involved in research and development activities to ensure the innovation and development of efficient products.
Some of the recent product launches and developments are as follows:
In July 2022, Verathon announced the release of the BFlex 2.8 Single-use Bronchoscope. BFlex single-use bronchoscopes are designed specifically for the critical needs of the ICU, OR, and ED. The BFlex 2.8 is Verathon's first single-use pediatric bronchoscope and delivers the drivability and image quality needed for pediatric bronchoscopies, airway management, and lung isolation procedures.
In May 2021, Olympus Corporation received FDA 510(k) clearance for its Airway Mobilescopes—MAF-TM2, MAF-GM2, and MAF-DM2—enabling providers to perform a variety of upper and lower airway management procedures.
In May 2021, Medtronic launched the SonarMed airway monitoring system to continuously check any endotracheal tube (ETT) obstruction and position for neonates and infants. It is the first and only device of its kind. The SonarMed airway monitoring system utilizes acoustic technology to check for ETT obstruction and verify position in real-time, giving clinicians vital information required to make more informed, life-saving decisions for their patients.
Thus, an increase in product development, launches, and approvals is likely to drive the airway management devices market in the near future.
Market Overview
The North America Airway management devices market is segmented into the US, Canada, and Mexico. The market growth in this region can be attributed to the increasing geriatric population and the rising prevalence of chronic respiratory diseases. The US is dominating the airway management market in North America. The continuously rising incidence of respiratory disorders is believed to fuel this market's future growth. Currently, asthma and chronic obstructive pulmonary disease (COPD) are major health burdens in the US. Asthma is a chronic inflammatory disorder due to airway narrowing and obstruction. COPD is due to obstruction of airflow from the lungs, which causes chronic inflammatory lung disease. Smoking is the main cause of COPD in the US. The increasing smoking in the US is driving the airway management devices market. According to the Centers for Disease Control and Prevention (CDC), in 2020, ~5.0% of adults were diagnosed with COPD, emphysema, or chronic bronchitis in the United States. Approximately 25 million people are affected with asthma, and 14.8 million have COPD. Further, COPD was the fourth leading cause of death in the United States in 2018. Furthermore, about 1 in every 10 infants born in 2020 were preterm births. Thus, the growing prevalence of chronic respiratory disorders and preterm births in the United States is driving the airway management devices market.
Strategic insights for the North America Airway Management Devices provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the North America Airway Management Devices refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.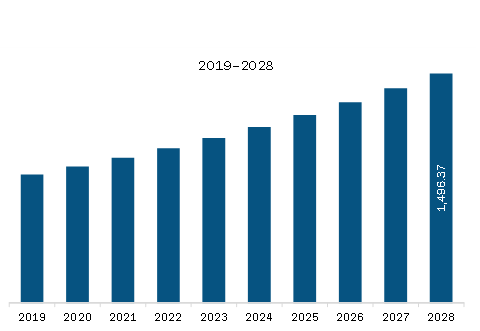
North America Airway Management Devices Strategic Insights
North America Airway Management Devices Report Scope
Report Attribute
Details
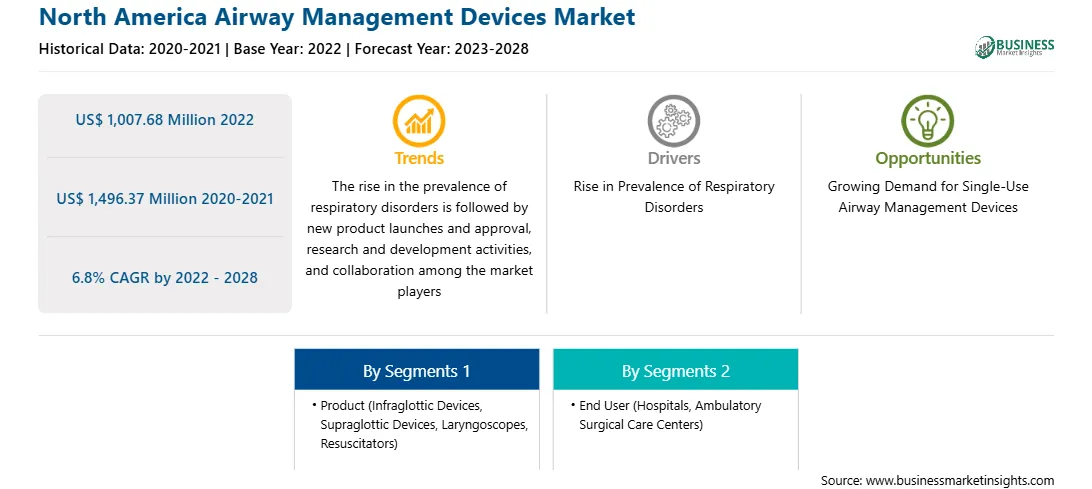
Market size in 2022
US$ 1,007.68 Million
Market Size by 2028
US$ 1,496.37 Million
Global CAGR (2022 - 2028)
6.8%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Product
By End User
Regions and Countries Covered
North America
Market leaders and key company profiles
North America Airway Management Devices Regional Insights
North America Airway Management Devices Market Segmentation
The North America airway management devices market is segmented into product, end user, and country.
Based on product, the market is segmented into infraglottic devices, supraglottic devices, laryngoscopes, resuscitators, and others. The infraglottic devices segment registered the largest market share in 2022.
Based on end user, the market is categorized into hospitals, ambulatory surgical care centers, and others. The hospitals segment held the largest market share in 2022.
Based on country, the market is segmented into the US, Canada, and Mexico. The US dominated the market in 2022.
Teleflex Incorporated; Smiths Medical; Medtronic; Intersurgical Ltd; Ambu A/S; Medline Industries, Inc.; Koninklijke Philips N.V.; ARMSTRONG MEDICAL; Mercury Medical; and Cook Medical LLC are among the leading companies operating in the airway management devices market in the region.
The North America Airway Management Devices Market is valued at US$ 1,007.68 Million in 2022, it is projected to reach US$ 1,496.37 Million by 2028.
As per our report North America Airway Management Devices Market, the market size is valued at US$ 1,007.68 Million in 2022, projecting it to reach US$ 1,496.37 Million by 2028. This translates to a CAGR of approximately 6.8% during the forecast period.
The North America Airway Management Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Airway Management Devices Market report:
The North America Airway Management Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Airway Management Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Airway Management Devices Market value chain can benefit from the information contained in a comprehensive market report.