The market's growth is attributed to key driving factors, such as the rising importance of medical waste minimization and increasing FDA approvals of reprocessed medical devices. However, the limitations of reprocessed medical devices and stringent regulatory requirements are restraining the market growth.
The demand for reprocessed medical devices increased rapidly due to the COVID-19 pandemic The Food and Drug Admninstration increased its approvals in response to soaring demand and shortages during the COVID-19 pandemic. For instance, in January 2022, Innovative Health, LLC, a leader in reprocessing single-use cardiology devices, obtained FDA clearance to reprocess Boston Scientific’s INTELLAMAP ORION High-Resolution Mapping Catheter. The catheter is widely utilized in catheter ablation techniques for atrial fibrillation (Afib). Similarly, in August 2020, Northeast Scientific Inc. received FDA clearance for reprocessing of .014 Digital Intravascular ultrasound (IVUS) catheter. Also, in July 2019, Innovative Health received the FDA approval to reprocess Biosense Webster’s PentaRay Nav Eco High-Density mapping catheter, which is crucial medical equipment in AFib practices. Hospitals employing the reprocessed PentaRay catheter could save thousands of dollars per AFib case, making it reasonable for a broader patient range. This system is used in processes involving His-bundle pacing (HBP), which helped the company generate extra revenue and strengthen its market position. Thus, the increasing FDA approvals mentioned above are the major factors creating further opportunities for the market.
Middle East and Africa Reprocessed Medical Devices Market Revenue and Forecast to 2028 (US$ Mn)
MIDDLE EAST AND AFRICA REPROCESSED MEDICAL DEVICES MARKET SEGMENTATION
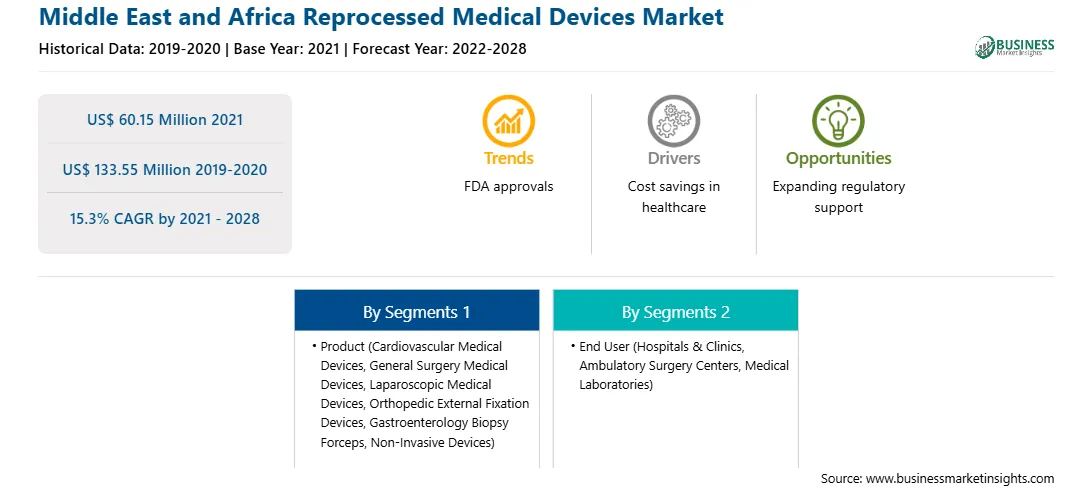
The Middle East and Africa reprocessed medical devices market, based on product, has been segmented into cardiovascular medical devices, gastroenterology biopsy forceps, orthopedic external fixation devices, laparoscopic medical devices, general surgery medical devices, non-invasive devices, and others. The Middle East and Africa reprocessed medical devices market, based on end user was segmented into hospitals and clinics, ambulatory surgical centers, medical laboratories, and others. Geographically, Middle East and Africa reprocessed medical devices market can be divided into UAE, Saudi Arabia, South Africa and Rest of Middle East and Africa.
Medline Industries, Inc., Arjo Medical Devices, Stryker Corporation, Teleflex Incorporated, Johnson and Johnson Services, Inc., 3M, Cardinal Health Inc and Vanguard AG are among the leading companies operating in the Middle East and Africa reprocessed medical devices market.
Strategic insights for the Middle East and Africa Reprocessed Medical Devices provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 60.15 Million |
| Market Size by 2028 | US$ 133.55 Million |
| Global CAGR (2021 - 2028) | 15.3% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | Middle East and Africa
|
| Market leaders and key company profiles |
The geographic scope of the Middle East and Africa Reprocessed Medical Devices refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Middle East and Africa Reprocessed Medical Devices Market is valued at US$ 60.15 Million in 2021, it is projected to reach US$ 133.55 Million by 2028.
As per our report Middle East and Africa Reprocessed Medical Devices Market, the market size is valued at US$ 60.15 Million in 2021, projecting it to reach US$ 133.55 Million by 2028. This translates to a CAGR of approximately 15.3% during the forecast period.
The Middle East and Africa Reprocessed Medical Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Middle East and Africa Reprocessed Medical Devices Market report:
The Middle East and Africa Reprocessed Medical Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Middle East and Africa Reprocessed Medical Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Middle East and Africa Reprocessed Medical Devices Market value chain can benefit from the information contained in a comprehensive market report.