Middle East and Africa Pharmacovigilance and Drug Safety Software Market
No. of Pages: 116 | Report Code: TIPRE00022772 | Category: Technology, Media and Telecommunications
No. of Pages: 116 | Report Code: TIPRE00022772 | Category: Technology, Media and Telecommunications
Pharmacovigilance (PV) plays an essential role in the healthcare system through assessment, monitoring, and finding of drug interactions and their effects in human. Pharmacovigilance helps companies to monitor any adverse drug reaction events during the trial phase and also during the post marketing period.
Thus, the globalization of pharmacovigilance are expected to create a significant demand for pharmacovigilance and drug safety software in the coming years, which is further anticipated to drive the pharmacovigilance and drug safety software market.
The Pharmacovigilance and Drug Safety Software market in this pandemic is on increasing due to the increased demand for treatments due to the outbreak of COVID-19. There is a current focus on drug safety in this area and how one can avoid counterfeit drugs from entering the supply chain. Methods for regional cooperation and expertise gained from more active projects should be improved. Technology must be used to improve the ability to report spontaneously and to make data available for processing. The countries mainly focused on the treatment of COVID-19 19 patients and hence the Pharmacovigilance and Drug Safety Software is facing impact. Based on the above-mentioned reason, the market of the Pharmacovigilance and Drug Safety Software is likely to impact.
Strategic insights for the Middle East and Africa Pharmacovigilance and Drug Safety Software provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
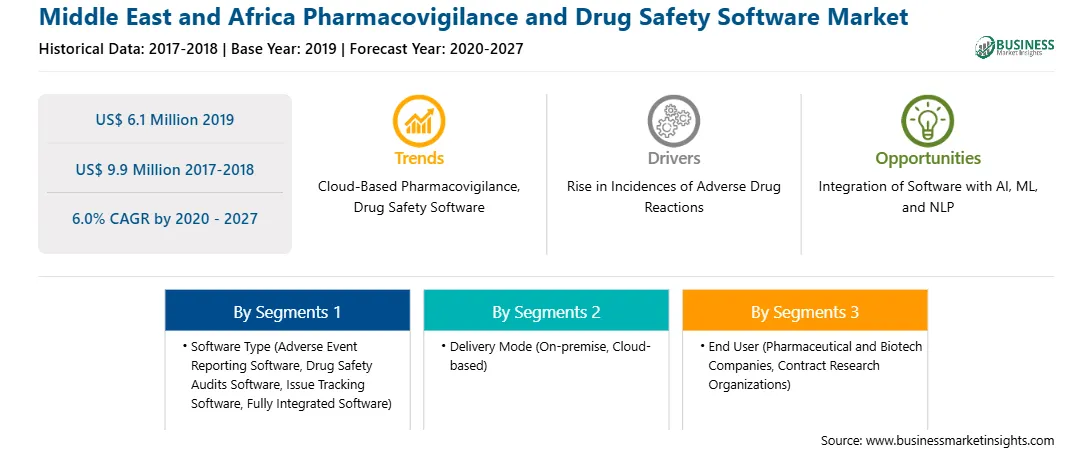
| Market size in 2019 | US$ 6.1 Million |
| Market Size by 2027 | US$ 9.9 Million |
| Global CAGR (2020 - 2027) | 6.0% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Software Type
|
| Regions and Countries Covered | Middle East and Africa
|
| Market leaders and key company profiles |
The geographic scope of the Middle East and Africa Pharmacovigilance and Drug Safety Software refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
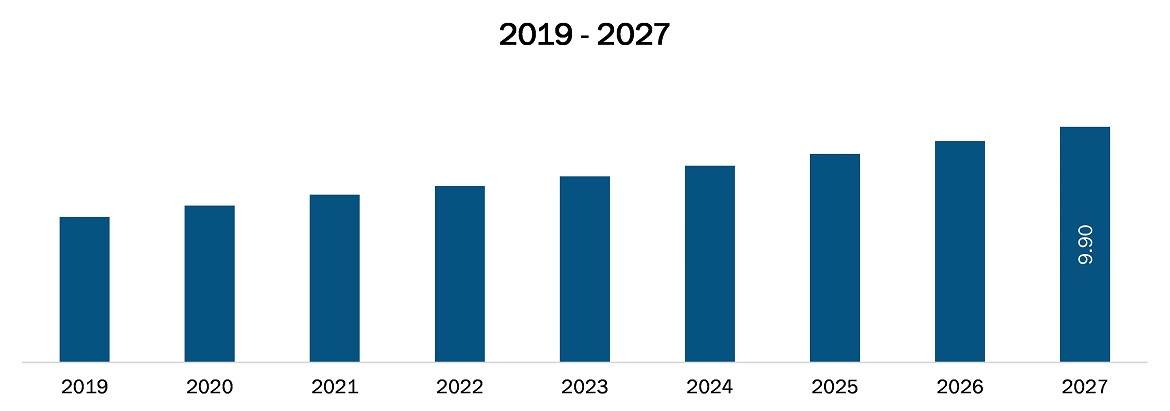
The pharmacovigilance and drug safety software market in Middle East and Africa is expected to grow from US$ 6.1 million in 2019 to US$ 9.9 million by 2027; it is estimated to grow at a CAGR of 6.0% from 2020 to 2027. Pharmacovigilance is the processes for monitoring and evaluating adverse drug reactions and it is a key component of effective drug regulation systems, clinical practice and public health programs. The number of Adverse Drug Reactions (ADRs) reported resulted in an increase in the volume of data handled. In the current network of pharmacovigilance centers coordinated by the Uppsala Monitoring Centre, pharmacovigilance is a critical and integral part of clinical research and these days it is growing in many countries. Today many pharmacovigilance centers are working for drug safety monitoring, however, at the turn of the millennium pharmacovigilance faces major challenges in aspect of better safety and monitoring of drugs. Thus, increasing the globalization in pharmacovigilance sector helps in drug safety software. It improves the quality and efficacy of pharmacovigilance and drug safety software and is expected to favor the growth of the market during the forecast period.
In terms of software type, the adverse event reporting software segment accounted for the largest share of the Middle East and Africa pharmacovigilance and drug safety software market in 2019. In terms of delivery mode, the on-premise segment held a larger market share of the pharmacovigilance and drug safety software market in 2019. In terms of end user, the contract research organizations segment held a larger market share of the pharmacovigilance and drug safety software market in 2019.
A few major primary and secondary sources referred to for preparing this report on the Pharmacovigilance and drug safety software market in Middle East and Africa are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Veeva Systems
By Software Type
By Delivery Mode
By End User
By Country
The Middle East and Africa Pharmacovigilance and Drug Safety Software Market is valued at US$ 6.1 Million in 2019, it is projected to reach US$ 9.9 Million by 2027.
As per our report Middle East and Africa Pharmacovigilance and Drug Safety Software Market, the market size is valued at US$ 6.1 Million in 2019, projecting it to reach US$ 9.9 Million by 2027. This translates to a CAGR of approximately 6.0% during the forecast period.
The Middle East and Africa Pharmacovigilance and Drug Safety Software Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Middle East and Africa Pharmacovigilance and Drug Safety Software Market report:
The Middle East and Africa Pharmacovigilance and Drug Safety Software Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Middle East and Africa Pharmacovigilance and Drug Safety Software Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Middle East and Africa Pharmacovigilance and Drug Safety Software Market value chain can benefit from the information contained in a comprehensive market report.