The growth of the market is due to are increasing prevalence of cancer and growing demand for next-generation antibody therapeutics. However, complications associated with the manufacturing and approvals of next-generation antibodies is expected to restrict the market growth during the forecast period.
Rising developments in biotechnology have led to increasing acceptance for next-generation antibodies therapeutics, which is further driving its use in autoimmune, inflammatory, and chronic treatment diseases among others. Next-generation antibody treatments have resulted from the application of sophisticated technologies in antibody therapeutics such as antibody–drug conjugates (ADCs), glycoengineered antibodies, and specific antibodies (BsAbs). Therefore, applications of next-generation antibodies are being widely studied to treat various chronic diseases. The rising demand of these antibodies have resulted in the rapid increase in the approval of ADCs and other next-generation antibodies therapeutics. For instance, in May 2020, Takeda Pharmaceutical Company Limited announced the FDA approval of ALUNBRIG (brigatinib) for adult patients with anaplastic lymphoma kinase-positive (ALK+) metastatic non-small cell lung cancer (NSCLC) as detected by an FDA-approved test. ALUNBRIG's current indication has been expanded to encompass the first-line setting with its approval. ALUNBRIG is a next-generation tyrosine kinase inhibitor (TKI) that is designed to target ALK molecular abnormalities. Similarly, in 2019, Genentech announced FDA accelerated approval of Polatuzumab vedotin-piiq, a CD79b-directed antibody-drug conjugate indicated in combination with bendamustine and a rituximab product for adult patients with relapsed or refractory diffuse large B-cell lymphoma.
Moreover, rising investments from biopharmaceutical and pharmaceutical companies for the manufacturing and development of these next-generation antibodies are driving the growth of the market. In addition, contract manufacturing and development organizations (CDMOs) forges a link to next-generation antibody conjugates to offer even more comprehensive ADC capabilities. CDMOs are developing site-specific linker technologies, expanding their payload options, and planning for indications other than cancer. For instance, in September 2020, MilliporeSigma has announced a US$ 65 million expansion of its highly potent active pharmaceutical ingredient (HPAPI) and active pharmaceutical ingredient production capabilities and capacity at its Madison (WI) location. Similarly, in July 2019, REGENXBIO and Neurimmune AG have signed a licensing, development, and commercialization collaboration to develop innovative AV gene treatments employing NAV vectors to deliver human antibodies against chronic neurodegenerative disorders such as tauopathies.
Therefore, the next-generation antibody market is anticipated to grow rapidly in the forecast period due to the rising clinical trials approvals and high adoption of next-generation antibodies therapeutics for the treatment of various diseases.
The socio-economic conditions in the Middle Eastern economies have been widely affected by the COVID–19 pandemic. The pandemic has restricted research and development activities in these countries. Since a considerable population already faces a growing burden of chronic diseases, the virus made them more vulnerable. However, economies in this region reacted wisely to the pandemic to avoid the spread of infection.
Similar to other regions, lockdown and social distancing measures were imposed in the MEA. Since these measures limited people's access to work, regular income, payments, schools, and healthcare, the MEA countries underwent huge losses and are still trying to recover them. Due to the less developed economies, most countries in this region depend on suppliers for healthcare products. A widespread outbreak of COVID-19 in Africa led to disruption in healthcare services, hindering access to healthcare supplies.
A COVID-19 vaccine is currently being developed by more than eight Iranian businesses and universities. While Iran does not suffer from the same poverty levels as other nations, sanctions generate other issues that jeopardize fair vaccine availability for its 80 million people, such as restrictions on Iranian participation in the COVAX facility as a self-financing member. Iran has boosted efforts to manufacture Covid-19 vaccine supplies locally in the last week. Coviran Barekat, the first coronavirus vaccine developed by researchers at Iran's Headquarters for Executing the Imam's Order, is currently undergoing phase 3 trials involving 20,000 patients. Saudi Arabia's Health Ministry has raised the number of appointments available for Pfizer-vaccination BioNTech's in Riyadh and Jeddah. Senior citizens and expatriates receive priority attention and can get vaccinated without a prior appointment. In addition, the Ministry of Health in Saudi Arabia has approved the use of Coronavirus vaccines produced by American businesses Johnson & Johnson and Moderna for incoming guests (MoH). Thus, constant research on COVID-19 using biotechnology is anticipated to propel the market over the next few years.
Strategic insights for the Middle East and Africa Next-generation Antibody provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
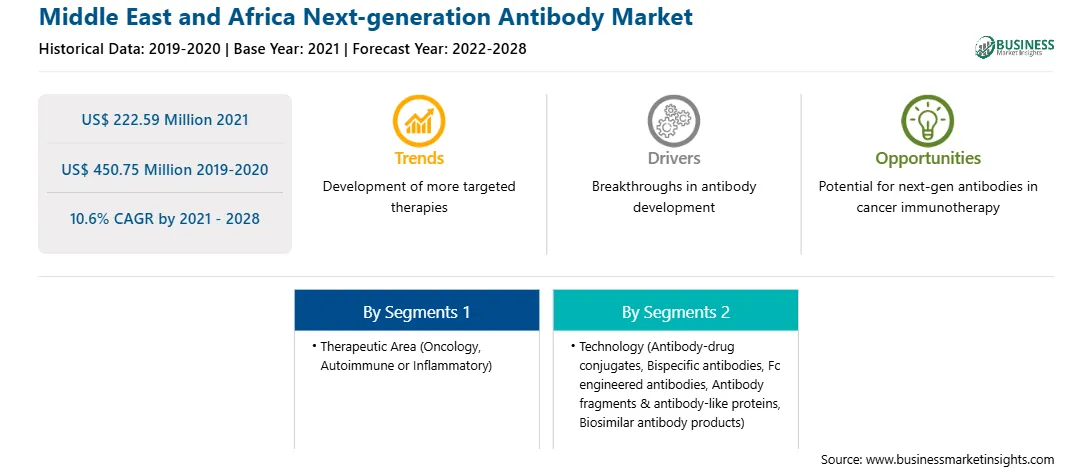
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 222.59 Million |
| Market Size by 2028 | US$ 450.75 Million |
| Global CAGR (2021 - 2028) | 10.6% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Therapeutic Area
|
| Regions and Countries Covered | Middle East and Africa
|
| Market leaders and key company profiles |
The geographic scope of the Middle East and Africa Next-generation Antibody refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
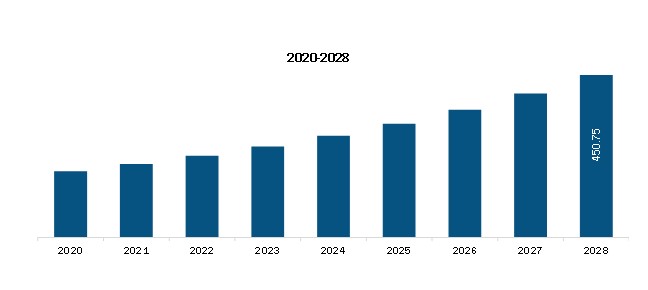
The Middle East and Africa Next-generation Antibody Market is valued at US$ 222.59 Million in 2021, it is projected to reach US$ 450.75 Million by 2028.
As per our report Middle East and Africa Next-generation Antibody Market, the market size is valued at US$ 222.59 Million in 2021, projecting it to reach US$ 450.75 Million by 2028. This translates to a CAGR of approximately 10.6% during the forecast period.
The Middle East and Africa Next-generation Antibody Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Middle East and Africa Next-generation Antibody Market report:
The Middle East and Africa Next-generation Antibody Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Middle East and Africa Next-generation Antibody Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Middle East and Africa Next-generation Antibody Market value chain can benefit from the information contained in a comprehensive market report.