Research and development (R&D) are fundamentally an important phase of preproduction activities. The former revolves around a planned experiment to review, analyze, and revise the accepted or established principles, and draw conclusions or rectify already known facts of knowledge if required. However, in the latter phase, the results of former activities are checked in practical application scenarios to check their reproducibility in the production phase by developing new or improved products. Although the performance of a product under investigation is often unknown or not optimized, compliance with the GMPs is critical for ensuring the safety and efficacy of investigational medicines. Notebook reviews, spot inspections, and varied training programs, among other methods, are used to ensure better compliance in analytical laboratories, wherein investigational medicines are tested before their release for clinical use. As electronic systems such as laboratory information management system (LIMS), electronic databases, and data acquisition software are an integral part of scientists’ work, they must be capable of the proper utilization of these systems. Notebook documentation, analytical instrumentation logs (calibration records and cross-references), method execution, laboratory operations, and electronic records are pivotal to ensuring GMPs. The compliance of all key actions and documents is assessed against current expected departmental work practices, instrument operating procedures (IOPs), and standard operating procedures (SOPs), and the findings are captured on standard forms. Thus, R&D departments are focusing on the use of IOPs and SOPs in their operations to avoid excessive monetary spending. Ensuring GMP compliance in R&D activities is essential to avoid product recalls, as it takes years to develop a product; product recalls may incur huge losses to manufacturers. Moreover, by opting for GMP testing services in R&D, companies can carry out proper research activities and develop products according to the needs of the GMP testing services market. Thus, the growing adoption of GMPs in R&D activities is expected to create notable opportunities for the growth of the Middle East & Africa GMP testing service market during the forecast period.
The United Arab Emirates (UAE), Saudi Arabia, and South Africa are the three major countries in the Middle East and South Africa. Saudi Arabia is the largest market for GMP testing services in this region in 2021. The GMP testing service market in the Middle East & Africa is anticipated to grow at a CAGR of 5.8% during the forecast period; this growth is attributed to the rising healthcare expenditure and increasing government initiatives in terms of funding and healthcare-related policies. Due to limited access to developed technologies in the healthcare, biotechnology, and pharmaceutical industries, the GMP testing service market is growing steadily in the rest of the Middle East and African countries.
Strategic insights for the Middle East & Africa GMP Testing Service provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
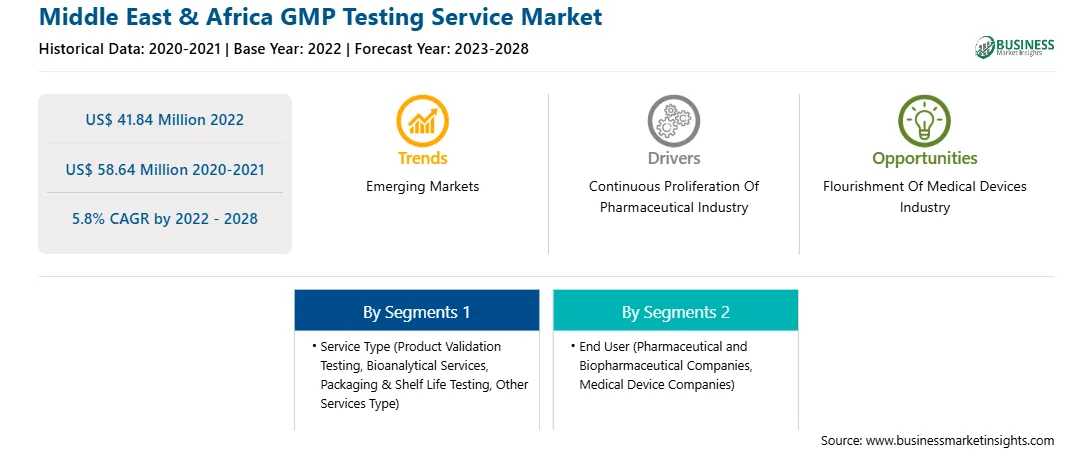
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 41.84 Million |
| Market Size by 2028 | US$ 58.64 Million |
| Global CAGR (2022 - 2028) | 5.8% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered | Middle East and Africa
|
| Market leaders and key company profiles |
The geographic scope of the Middle East & Africa GMP Testing Service refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Middle East & Africa GMP testing service market is segmented on the basis of service type, end user, and country. Based on service type, the Middle East & Africa GMP testing service market is segmented into product validation testing, bioanalytical services, packaging & shelf-life testing, and other services type. The product validation testing segment held the largest market share in 2022.
Based on end user, the Middle East & Africa GMP testing service market is bifurcated into pharmaceutical and biopharmaceutical companies and medical device companies. The pharmaceutical and biopharmaceutical companies segment held a larger market share in 2022.
Based on country, the Middle East & Africa GMP testing service market is segmented into Saudi Arabia, South Africa, UAE, and the Rest of Middle East & Africa. The Rest of Middle East & Africa dominated the market share in 2022.
Eurofins Scientific; Almac Group; INTERTEK GROUP PLC; WuXi AppTec; Sartorius AG; Nelson Laboratories, LLC.; and Thermo Fisher Scientific Inc. (PPD Inc.) are among the leading companies operating in the Middle East & Africa GMP testing service market.
The Middle East & Africa GMP Testing Service Market is valued at US$ 41.84 Million in 2022, it is projected to reach US$ 58.64 Million by 2028.
As per our report Middle East & Africa GMP Testing Service Market, the market size is valued at US$ 41.84 Million in 2022, projecting it to reach US$ 58.64 Million by 2028. This translates to a CAGR of approximately 5.8% during the forecast period.
The Middle East & Africa GMP Testing Service Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Middle East & Africa GMP Testing Service Market report:
The Middle East & Africa GMP Testing Service Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Middle East & Africa GMP Testing Service Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Middle East & Africa GMP Testing Service Market value chain can benefit from the information contained in a comprehensive market report.