In decentralized clinical trials (DCT), patients' physical access to hospital-based trial sites is reduced or eliminated. In DCTs, digital technologies are used to enable access of patients to clinical research, remote data collection and monitoring, and communication between investigators and participants. A hybrid clinical trial approach combines home-based and on-site activities, bringing the best patient experience and meeting complex protocol regimes, gaining traction across various therapeutic areas and trial phase journeys. Initially, due to challenges such as patient privacy, data security, regulatory barriers, and complex protocol regimes, the adoption of DCT was affected. However, due to the COVID-19 pandemic, the sponsors of clinical trials adopted decentralized and hybrid clinical techniques for developing drugs. They could not continue with traditional trials as they required in-person visits. Due to the restrictions for travel, the only way to gather data and keep trials going was to work remotely and adopt technology much faster than they would have otherwise. Therefore, decentralization broadens trial access to reach a larger number and potentially a more diverse pool of patients.
Further, hybrid clinical trials allow sponsors to strategically incorporate decentralized clinical trial (DCT) elements into study designs. These trial models offer unprecedented flexibility, so more companies are interested in hybrid trials, and the resulting growth is redefining the industry landscape. According to ObvioHealth, the FDA plans to unveil protocols in 2023 to support the use of DCT methods, inspiring confidence in these components for future clinical research. Thus, the increasing focus on using decentralized and hybrid clinical trials over traditional clinical trial methods is expected to provide lucrative opportunities for the clinical trials market during the forecast period.
The clinical trials market in the Middle East & Africa is segmented into Saudi Arabia, the UAE, South Africa, and the Rest of Middle East & Africa. The regional growth is attributed to rising rate of cancer and investments in developing healthcare infrastructure. Factors such as growing awareness of the advantages of clinical trials, government showing a propensity to spend on healthcare infrastructure and research are likely to further propel the clinical trials market growth during the forecast period.
Strategic insights for the Middle East & Africa Clinical Trials provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Middle East & Africa Clinical Trials refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Middle East & Africa Clinical Trials Strategic Insights
Middle East & Africa Clinical Trials Report Scope
Report Attribute
Details
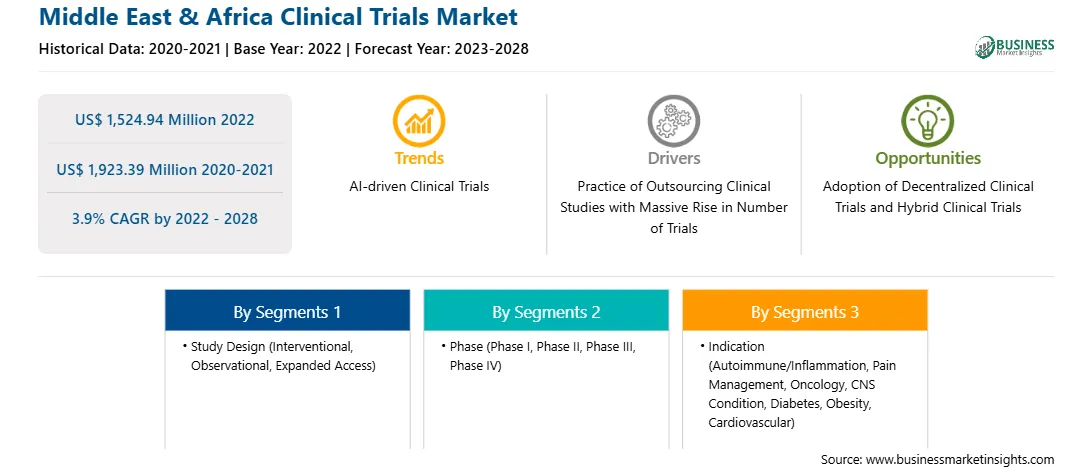
Market size in 2022
US$ 1,524.94 Million
Market Size by 2028
US$ 1,923.39 Million
Global CAGR (2022 - 2028)
3.9%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Study Design
By Phase
By Indication
Regions and Countries Covered
Middle East and Africa
Market leaders and key company profiles
Middle East & Africa Clinical Trials Regional Insights
Middle East & Africa Clinical Trials Market Segmentation
The Middle East & Africa clinical trials market is segmented into phase, study design, indication, and country.
Based on phase, the Middle East & Africa clinical trials market is segmented into phase I, phase II, phase III, and phase IV. The phase III segment registered the largest Middle East & Africa clinical trials market share in 2022.
Based on study design, the Middle East & Africa clinical trials market is segmented into interventional, observational, and expanded access. The interventional segment held the largest Middle East & Africa clinical trials market share in 2022.
Based on indication, the Middle East & Africa clinical trials market is segmented into autoimmune/inflammation, pain management, oncology, CNS condition, diabetes, obesity, cardiovascular, and others. The oncology segment held the largest Middle East & Africa clinical trials market share in 2022.
Based on country, the Middle East & Africa clinical trials market has been categorized into Saudi Arabia, South Africa, the UAE, and the Rest of Middle East & Africa. Saudi Arabia dominated the Middle East & Africa clinical trials market share in 2022.
ICON Plc; IQVIA Holdings Inc; Laboratory Corp of America Holdings; Parexel International Corp; SGS SA; Syneos Health Inc; Thermo Fisher Scientific Inc; and WuXi AppTec Co Ltd. are some of the leading companies operating in the Middle East & Africa clinical trials market.
The Middle East & Africa Clinical Trials Market is valued at US$ 1,524.94 Million in 2022, it is projected to reach US$ 1,923.39 Million by 2028.
As per our report Middle East & Africa Clinical Trials Market, the market size is valued at US$ 1,524.94 Million in 2022, projecting it to reach US$ 1,923.39 Million by 2028. This translates to a CAGR of approximately 3.9% during the forecast period.
The Middle East & Africa Clinical Trials Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Middle East & Africa Clinical Trials Market report:
The Middle East & Africa Clinical Trials Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Middle East & Africa Clinical Trials Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Middle East & Africa Clinical Trials Market value chain can benefit from the information contained in a comprehensive market report.