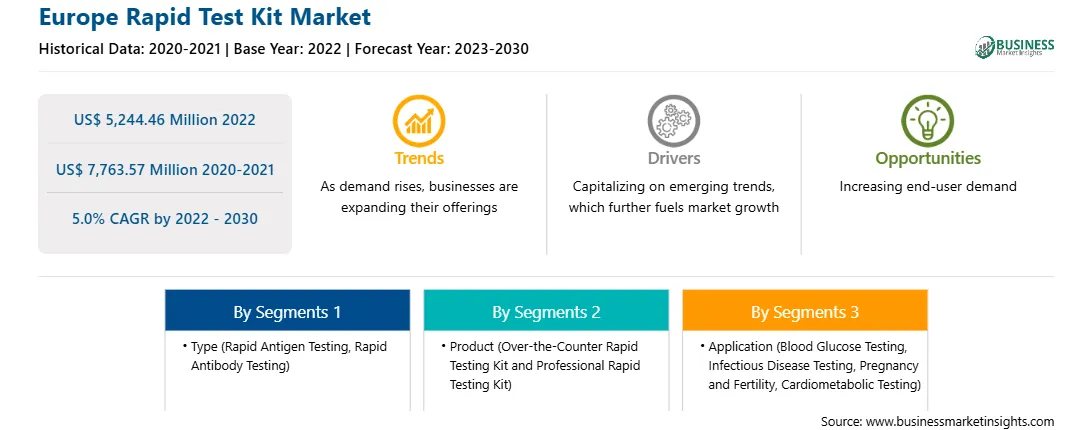
The Europe rapid test kit market was valued at US$ 5,244.46 million in 2022 and is expected to reach US$ 7,763.57 million by 2030; it is estimated to record a CAGR of 5.0% from 2022 to 2030. Strategic Initiatives by Manufacturers Drive Europe Rapid Test Kit Market
Manufacturers in the rapid test kits market emphasize on the adoption of strategies such as innovations, and product launches and approvals to maintain a competitive edge in the market. Such strategic initiatives by companies in the rapid test kits market benefit the overall market.
In 2022, Singapore-based diagnostics manufacturer INEX Innovate, a pioneer in the women's and fetal health sector in Asia, has received a CE mark for OvaCis Rapid Test, its flagship solution for ovarian cancer. By the end of 2022, OvaCis-a first-of-its-kind point-of-care (POCT) test that distinguishes benign from malignant ovarian cysts in an operating room setting-is expected to make its debut in the EU and Southeast Asian markets.
In January 2022, OraSure Technologies, Inc., a global leader in point-of-care and home diagnostic testing as well as sample collection technologies, launched the OraQuick HIV Self-Test, an oral swab-based in-home test for HIV-1 and HIV-2, for the European market. The test is accessible in six European countries: the UK, France, Germany, Italy, Spain, and Portugal.
In 2021, following the issuance of the CE certificate, FUJIFILM Europe GmbH was authorized to apply the CE mark and introduce an antigen test kit for the diagnosis of SARS-CoV-2 infection. The technique being utilized by Fujifilm is called proprietary silver amplification immunochromatography, and it is based on the same technology that is used to amplify the photos to be developed.
In December 2020, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for a virtually guided BinaxNOW COVID-19 Ag Card (by Abbott) rapid test, indicated for the at-home use for detection of COVID-19. BinaxNOW is a widely distributed and one of the most affordable rapid tests, which provides results in minutes.
In July 2020, the FDA granted Emergency Use Authorization (EUA) for a rapid, point-of-care, SARS-CoV-2 diagnostic test for use with its broadly available BD Veritor Plus System. The new assay generates results in 15 minutes on an easy-to-use, highly portable instrument. The test was meant to improve access to COVID-19 diagnostics by providing real-time results and aiding quick decision-making while the patient is still in the testing facility.Europe Rapid Test Kit Market Overview
The prevalence of the diabetes in Germany has been increasing significantly. According to the International Diabetes Federation, in 2021 ~62 million people were having diabetes. Moreover, according to the European Center for Disease Prevention and Control report, 8,641 malarial cases were registered in the European Union (EU) in 2019, of which 8,638 (>99%) were confirmed diagnoses. In September 2022, Germany signed an agreement worth US$ 1.34 billion with The Joint United Nations Programme on HIV/AIDS (UNAIDS) aiming to fight AIDS, tuberculosis, and malaria; with this, the country raised its contribution by 30% compared to 2019. Further, as per the endmalaria.org 2020 report, international sources provided 69% of total funding aiming for malaria control and elimination. The total funding for malaria control and elimination accounted for US$ 3.3 billion, including US$ 0.2 billion contributed by Germany. Thus, the rapid test kits market is likely to grow in Germany in the coming years with the rising prevalence of infectious disease such as diabetes, AIDS, tuberculosis, and malaria.
Europe Rapid Test Kit Market Revenue and Forecast to 2030 (US$ Million)
Strategic insights for the Europe Rapid Test Kit provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Europe Rapid Test Kit refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Europe Rapid Test Kit Strategic Insights
Europe Rapid Test Kit Report Scope
Report Attribute
Details
Market size in 2022
US$ 5,244.46 Million
Market Size by 2030
US$ 7,763.57 Million
Global CAGR (2022 - 2030)
5.0%
Historical Data
2020-2021
Forecast period
2023-2030
Segments Covered
By Type
By Product
By Application
By End User
Regions and Countries Covered
Europe
Market leaders and key company profiles
Europe Rapid Test Kit Regional Insights
Europe Rapid Test Kit Market Segmentation
The Europe rapid test kit market is segmented based on type, product, technology, application, end user, and country. Based on type, the Europe rapid test kit market is segmented into rapid antigen testing, rapid antibody testing, and others. The rapid antigen testing segment held the largest market share in 2022.
In terms of product, the Europe rapid test kit market is bifurcated into over-the-counter rapid testing kit and professional rapid testing kit. The over-the-counter rapid testing kit segment held a larger market share in 2022.
By technology, the Europe rapid test kit market is segmented into lateral flow assay, solid phase, agglutination, immunospot assay, and cellular component-based. The lateral flow assay segment held the largest market share in 2022.
Based on application, the Europe rapid test kit market is categorized into blood glucose testing, infectious disease testing, pregnancy and fertility, cardiometabolic testing, and others. The blood glucose testing segment held the largest market share in 2022.
In terms of end user, the Europe rapid test kit market is segmented into hospital and clinics, home care, diagnostics centers, and others. The hospital and clinics segment held the largest market share in 2022.
Based on country, the Europe rapid test kit market is segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe. Germany dominated the Europe rapid test kit market share in 2022.
F. Hoffmann-La Roche Ltd, Becton Dickinson and Co, ARKRAY Inc, Sysmex Partec GmbH, Fujirebio Europe NV, bioMerieux SA, Cepheid, Meril Life Sciences Pvt Ltd, QIAGEN NV, OraSure Technologies Inc, Guangzhou Wondfo Biotech Co Ltd, Denka Co Ltd, Abbott Laboratories, Trinity Biotech Plc, Premier Medical Corp Pvt Ltd, Bio-Rad Laboratories Inc, Hologic Inc, DiaSorin SpA, and Beckman Coulter Inc are some of the leading players operating in the Europe rapid test kit market.
1. F. Hoffmann-La Roche Ltd
2. Becton Dickinson and Co
3. Sysmex Partec GmbH
4. ARKRAY Inc
5. Fujirebio Europe NV
6. bioMerieux SA
7. Cepheid
8. Meril Life Sciences Pvt Ltd
9. QIAGEN NV
10. OraSure Technologies Inc
11. Guangzhou Wondfo Biotech Co Ltd
12. Denka Co Ltd
13. Abbott Laboratories
14. Trinity Biotech Plc
15. Bio-Rad Laboratories Inc
16. Hologic Inc
17. Premier Medical Corp Pvt Ltd
18. DiaSorin SpA
19. Beckman Coulter Inc
The Europe Rapid Test Kit Market is valued at US$ 5,244.46 Million in 2022, it is projected to reach US$ 7,763.57 Million by 2030.
As per our report Europe Rapid Test Kit Market, the market size is valued at US$ 5,244.46 Million in 2022, projecting it to reach US$ 7,763.57 Million by 2030. This translates to a CAGR of approximately 5.0% during the forecast period.
The Europe Rapid Test Kit Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Rapid Test Kit Market report:
The Europe Rapid Test Kit Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Rapid Test Kit Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Rapid Test Kit Market value chain can benefit from the information contained in a comprehensive market report.