Market Introduction
Point-of-care molecular diagnostics include portable devices, and assays & kits used to detect and diagnose diseases in human samples, such as throat swab, blood, serum, and stool. Molecular diagnostics are shifting from centralized laboratories to decentralized point-of-care molecular testing. Due to its simplicity, convenience, rapid turnaround time, and potential to improve patient outcomes, POCT is rapidly gaining traction.
Moreover, the growing demand for specific viral detection methods that consume less time for timely infection control is expected to bolster the market growth during the forecast period.
In Europe, currently, the UK and Russia are the hardest-hit countries by the COVID-19 pandemic, followed by France, Spain, Italy, and Germany. The implementation of drastic measures and travel restrictions, including closing the borders, impacted the import and export of products, especially in the first half of 2020. The continuous spread of the disease has led to an elevated demand for advanced diagnostic solutions in Europe, thus boosting the adoption of point-of-care molecular diagnostic kits. Moreover, uninterrupted investments and business activities by industry players are also boosting the point-of-care molecular diagnostic market growth. In 2020, European Medicines Agency (EMA) approved ~97 medicines for the marketing authorization, of which 39 were novel drugs. In October 2020, Eurofins introduced PCR-based COVID-19 diagnostic tests with at-home, self-sampling options, in the for European market. In January 2020, European Union (EU) commission announced an emergency call, through which it awarded US$ 55.64 million to 18 research projects. Among these, 3 of the projects are receiving a total of US$ 7.39 million to develop effective, rapid point-of-care diagnostics.
Market Overview and Dynamics
The Europe Point-of-Care Molecular Diagnostics market is expected to reach US$ 1,524.4 million by 2028 from US$ 653.5 million in 2021. The market is estimated to grow at a CAGR of 12.9% from 2021–2028. The rise of connected consumer electronic gadgets has transformed entire sectors of the economy, including healthcare. Patients and consumers are adjusting their interactions with POC medical product & services owing to the penetration of mobile devices. Moreover, with the rise of connected gadgets, consumers are transforming the way they manage and take control of their health. Therefore, manufacturers are developing easy to use, handy, and affordable POC molecular diagnostic devices for non-laboratory settings to meet increasing consumer demand. POC molecular diagnostic and monitoring devices are projected to become commonplace in the future across residential settings in low and middle-income economies owing to the rapidly emerging technological landscape and rising penetration of smartphones and connected devices.
Key Market Segments
In terms of product & services, the assays and kits segment accounted for the largest share of the Europe Point-of-Care Molecular Diagnostics market in 2020. In terms of technology, the PCR segment accounted for the largest share of the Europe Point-of-Care Molecular Diagnostics market in 2020. In terms of application, the infectious diseases segment accounted for the largest share of the Europe Point-of-Care Molecular Diagnostics market in 2020. In terms of end user, the diagnostic laboratories segment accounted for the largest share of the Europe Point-of-Care Molecular Diagnostics market in 2020.
Major Sources and Companies Listed
A few major primary and secondary sources referred to for preparing this report on the Point-of-Care Molecular Diagnostics market in Europe are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are bioMérieux SA, F. Hoffmann-La Roche Ltd., Danaher Corporation, Enzo Biochem, Inc., Abbott, binx health, Inc., Meridian BioScience, Inc., Biocartis, Quidel Corporation, and Bio-Rad Laboratories, Inc.
Reasons to Buy Report
EUROPE POINT-OF-CARE MOLECULAR DIAGNOSTICS MARKET SEGMENTATION
By Technology
By Application
By End User
By Country
• Germany
• UK
• France
• Spain
• Italy
• Rest of Europe
Companies Mentioned
Strategic insights for the Europe Point-of-Care Molecular Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
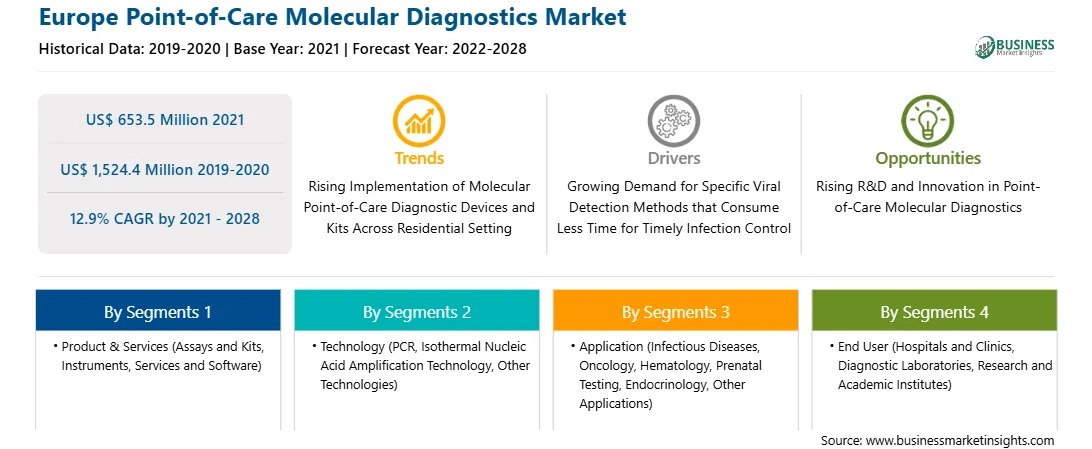
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 653.5 Million |
| Market Size by 2028 | US$ 1,524.4 Million |
| Global CAGR (2021 - 2028) | 12.9% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product & Services
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Point-of-Care Molecular Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Europe Point-of-Care Molecular Diagnostics Market is valued at US$ 653.5 Million in 2021, it is projected to reach US$ 1,524.4 Million by 2028.
As per our report Europe Point-of-Care Molecular Diagnostics Market, the market size is valued at US$ 653.5 Million in 2021, projecting it to reach US$ 1,524.4 Million by 2028. This translates to a CAGR of approximately 12.9% during the forecast period.
The Europe Point-of-Care Molecular Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Point-of-Care Molecular Diagnostics Market report:
The Europe Point-of-Care Molecular Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Point-of-Care Molecular Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Point-of-Care Molecular Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.