Europe is the second-largest market for point of care diagnostics, and the UK, Germany, France, Italy, Spain, and rest of Europe are the major contributors to this market in this region. The growth of the market in Europe is characterized by the rise in the demand for advanced diagnostic solutions, rising prevalence of infectious and chronic conditions, and increase in the investment for research and development. Also, the presence of well-established manufacturing infrastructure for advanced diagnosis products is likely to propel the growth of the Europe point of care diagnostics market during the forecast period. Germany led the market. The government of Germany is putting efforts to improve the medical conditions for the people suffering from various health conditions. For instance, in November 2017, Lilly Germany developed the Diabetes Work initiative. The initiative was launched to spread the awareness regarding diabetes, which is expected to offer a lucrative opportunity for adoption of diagnostic kits. Further, in September 2018, the Germany government gave permission to market and sale OTC HIV home testing kits. Such constructive developments are projected to drive the growth of Europe point of care diagnostics market during the forecast period.
In case of COVID-19, Europe is highly affected specially France. The European economy is severely affected due to the exponential growth of COVID-19 cases in the region. Spain, Italy, Germany, France, and the UK are among the most affected European countries and number of deaths are also high. The current coronavirus (COVID-19) outbreak has been declared by WHO as a Public Health Emergency of International Concern, according to the International Health Regulation. Increasing number of coronavirus infections and demand for advanced diagnostic solutions is offering a favorable opportunity for adoption of point of care diagnostic kits in the region. Moreover, rising investments and business activities by industry players is also boosting the market even in pandemic situations. In 2020, European Medicines Agency (EMA) approved about 97 medicines for marketing authorization of which, 39 are novel drugs. Similarly, in October 2020, Eurofins introduces COVID-19 PCR tests with at-home self-sampling options in Europe region. Moreover, in October 2020, Siemens Healthineers launched rapid antigen test for diagnosis of COVID-19 in European countries. The CLINITEST rapid COVID-19 test is point of care cassette test that offers test result in 15 minutes. In addition, in April 2021, QIAGEN announced launch of Prep&Amp technology in order accelerate the COVID-19 diagnosis in Europe. The kit has received CE approval for marketing and distribution in Europe, also, the company has submitted its product for EUA (Emergency Use Application) in US. Such activities are likely to have constructive impact on market growth.
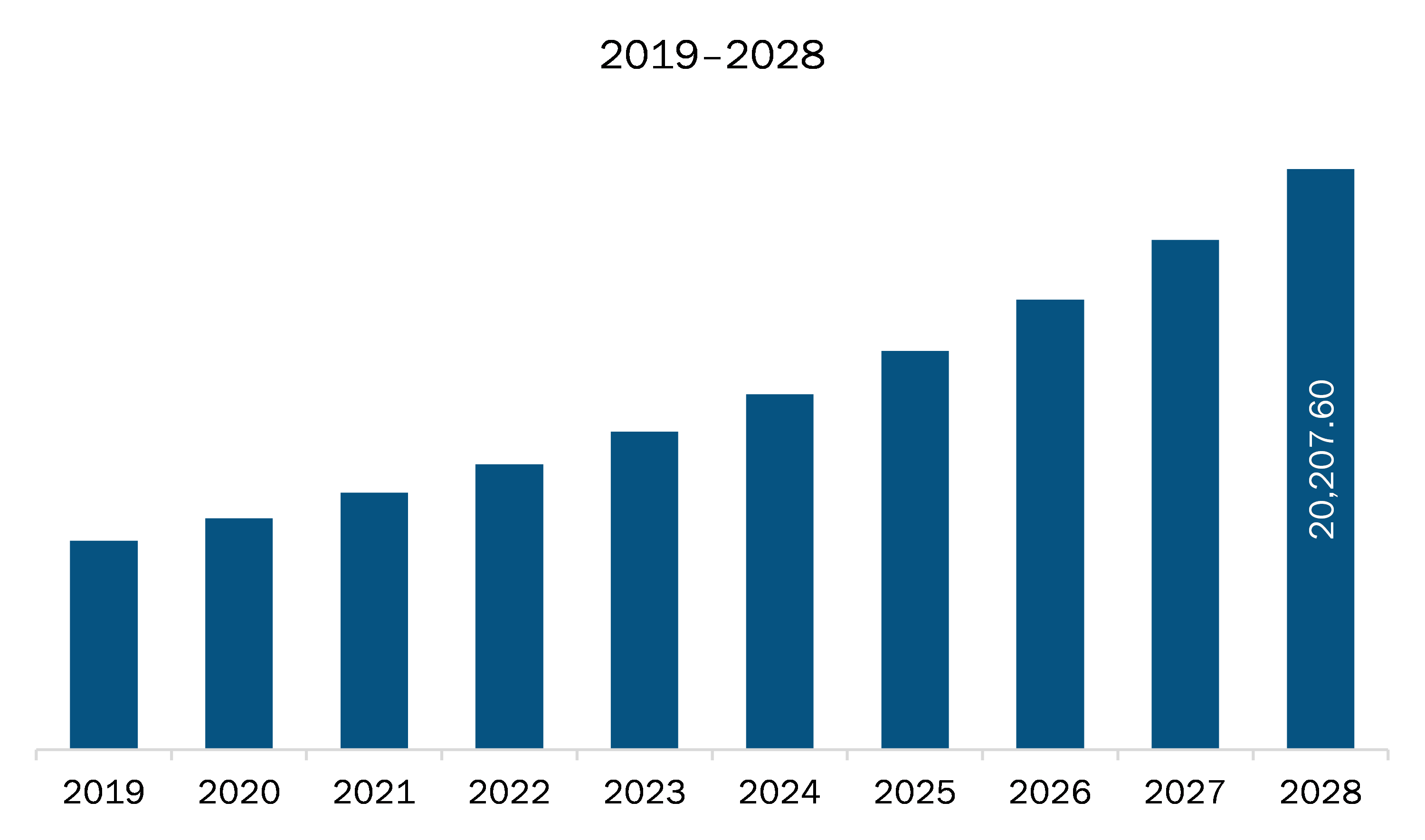
Strategic insights for the Europe Point of Care Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
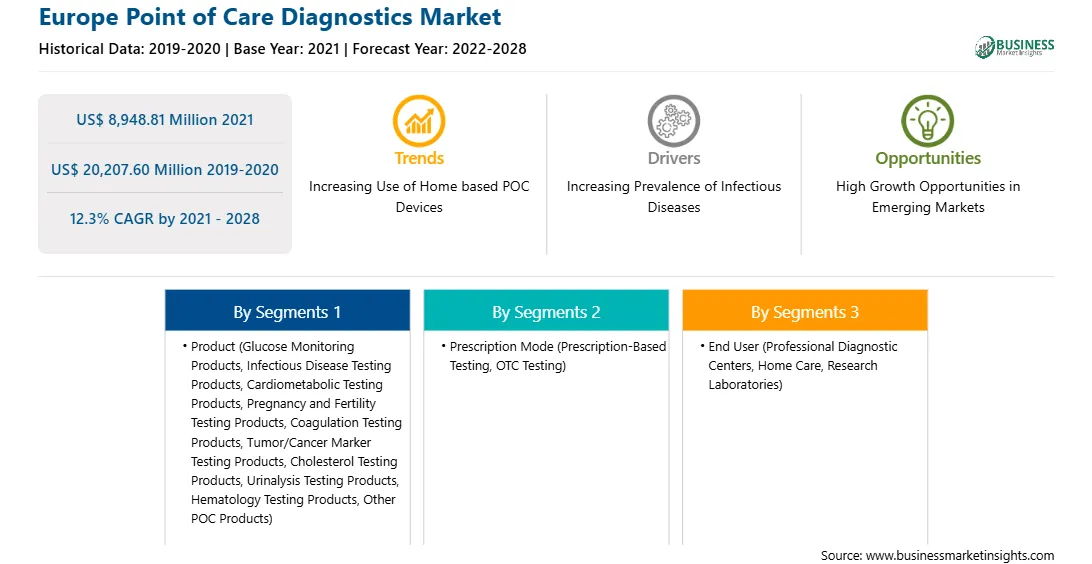
| Market size in 2021 | US$ 8,948.81 Million |
| Market Size by 2028 | US$ 20,207.60 Million |
| Global CAGR (2021 - 2028) | 12.3% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Point of Care Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Europe point of care diagnostics market is expected to grow from US$ 8,948.81 million in 2021 to US$ 20,207.60 million by 2028; it is estimated to grow at a CAGR of 12.3% from 2021 to 2028. Burgeoning use of home-based poc devices is expected to escalate the market growth. The increasing awareness about point of care testing is bringing the test conveniently and quickly to the patient at home. Most clinicians recognize POC testing as a requirement for early detection of life-threatening situations because laboratory results can be made available in real-time. POC diagnostic and monitoring devices are anticipated to become universal in-home setups as well as at doctor’s offices or hospitals, in low- and middle-income countries. Consumers are frequently using smartphones as platform devices. The health and monitoring applications of smartphones commonly include imaging, sensing, and diagnostics. Although glucose meters are widely used in home settings by diabetic patients, they can also be repurposed to perform common clinical assays such as immunoassays, molecular diagnostics, and enzymatic assays. The rise in geriatric population has resulted in increased expenditure on healthcare for health checkups, disease diagnoses, and treatments. The most constant health problems affecting this population include digestive disorders, cardiovascular diseases, bone and joint diseases, diabetes, and different types of cancer. The people of this age group claim diagnostic testing on a regular basis, owing to their low immunity and metabolism. Therefore, these factors have increased the demand of POCs for diagnostic purposes across Europe region.
In terms of product, the glucose monitoring products segment accounted for the largest share of the Europe point of care diagnostics market in 2020. In terms of prescription mode, the prescription-based testing segment held a larger market share of the Europe point of care diagnostics market in 2020. Further, the professional diagnostic centers segment held a larger share of the Europe point of care diagnostics market based on end user in 2020.
A few major primary and secondary sources referred to for preparing this report on the Europe point of care diagnostics market are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Abbott; BD; bioMerieux SA; BIO-RAD LABORATORIES INC.; Danaher; F. HOFFMANN-LA ROCHE LTD.; Johnson and Johnson Services, Inc.; Nova Biomedical; Polymer Technology Systems, Inc. (PTS); and Siemens AG.
The Europe Point of Care Diagnostics Market is valued at US$ 8,948.81 Million in 2021, it is projected to reach US$ 20,207.60 Million by 2028.
As per our report Europe Point of Care Diagnostics Market, the market size is valued at US$ 8,948.81 Million in 2021, projecting it to reach US$ 20,207.60 Million by 2028. This translates to a CAGR of approximately 12.3% during the forecast period.
The Europe Point of Care Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Point of Care Diagnostics Market report:
The Europe Point of Care Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Point of Care Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Point of Care Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.