Europe is the second-largest market for pediatric medical devices in the world owing to increasing demand for medical devices, growing research, and development in the field of medical technology, rising numbers of hospitals that are dedicated pediatric facilities. Likewise, rising funding for pediatric medical device innovation and the emergence of artificial intelligence, 3D printing, and medical robotics is likely to support the market growth during the forecast period. Technology plays an important role in the growth of this market, thus encouraging manufacturers to develop innovative and technologically pediatric advanced devices.
The European market is severely hit due to the exponential increase in COVID-19 cases in the region. Many nations are now reporting more patients per day than during the first wave earlier this year. Lockdowns are being reintroduced in the UK, Spain, and Italy, and Ireland. Due to the second wave of COVID-19 crisis, the governments of many countries are heading toward mounting up testing capacity. The European countries were profoundly affected due to the COVID-19 pandemic. Countries such as Italy, Spain, and France have recorded the most significant number of positive cases and have registered the maximum number of deaths. The rising rate of COVID-19 cases has increased stress on the region's healthcare system, thereby propelling the demand for diagnostic tests in its healthcare system and supporting the expansion of the sector in this region. Moreover, regulatory bodies in the region are taking preventive measures such as shutting down business operations. In March 2020, the European Medicines Agency established a managing committee to deal with the impact of COVID-19 on the supply chain of medicines. Many studies revealed the clinical features of COVID-19 in adults and infants. Limited knowledge about characteristics, results, and intrauterine transmission potential in pediatric aged 28 days or less have chances to acquire COVID-19. Due to the fear of infection in babies, people are refraining from hospitals, gynecology clinics, and baby care centers for neonatal care. These measures are likely to hamper the overall manufacturing and marketing of pediatric medical devices, which will have an adverse impact on market growth.
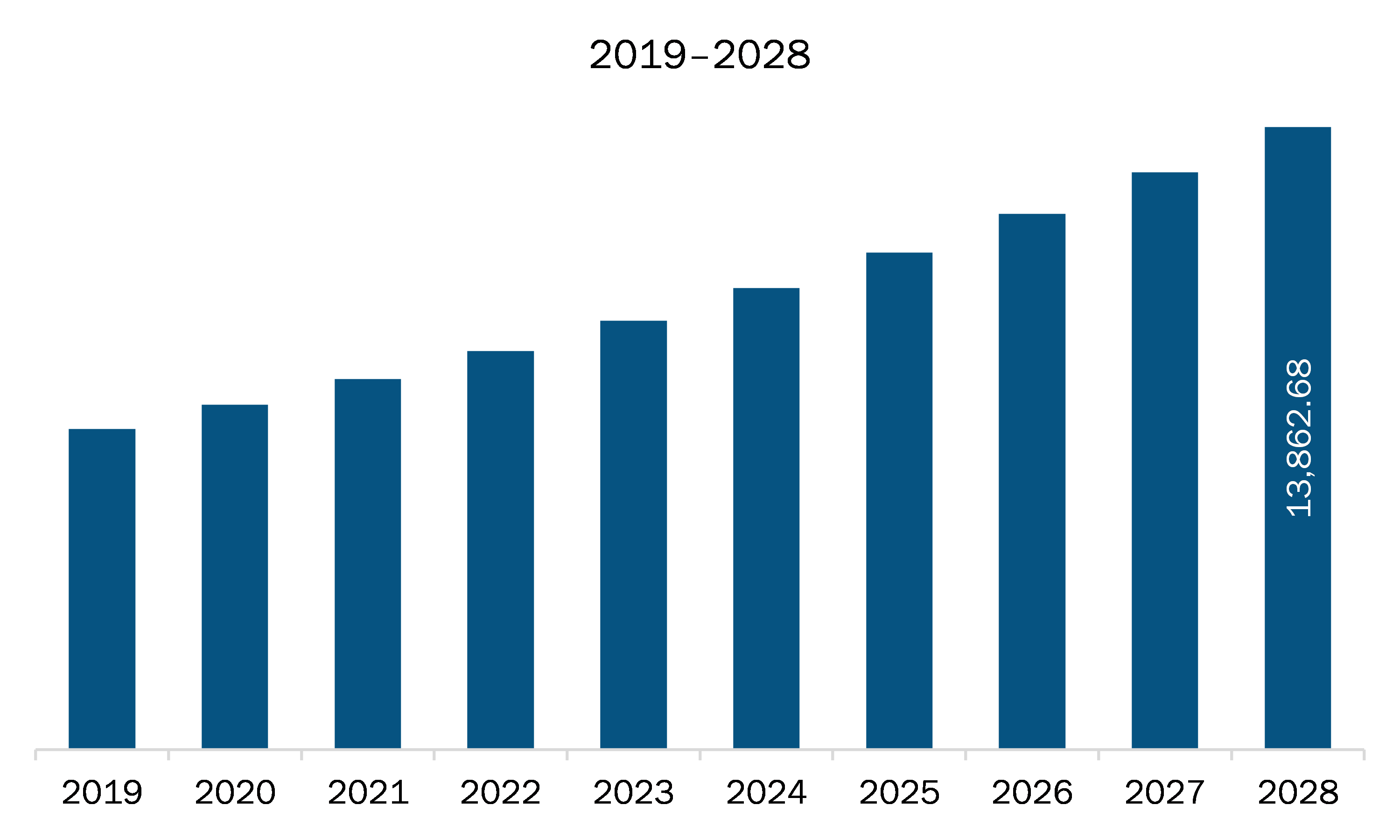
Strategic insights for the Europe Pediatric Medical Devices provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.

| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 8,253.86 Million |
| Market Size by 2028 | US$ 13,862.68 Million |
| Global CAGR (2021 - 2028) | 7.7% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Pediatric Medical Devices refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The pediatric medical devices market in Europe is expected to grow from US$ 8,253.86 million in 2021 to US$ 13,862.68 million by 2028; it is estimated to register a CAGR of 7.7% from 2021 to 2028. The rest of Europe in pediatric medical devices comprises Switzerland, Netherlands, Ireland, Belgium, Denmark, and others. Increasing demand for advanced healthcare facilities, rising adoption of pediatric medical devices and commercialization of novel interventional solutions are major factors boosting the pediatric medical devices market. With the rising healthcare facilities, the mortality rate among infants, children have rapidly decreased. For instance, according to the report- “State of Child Health 2020” by Royal College of Pediatrics and Child Health, the infant mortality rate (per 1,000 live births) has decreased 4.8 to 4.2 from 2014 to 2020 in Northern Ireland. Moreover, in 2017, there were 2 pediatric consultants per 10,000 children and young people (CYP) in Northern Ireland, 2.0 per 10,000 CYP in Wales and 2.2 per 10,000 CYP in Scotland which depicts the lack of pediatric consultant in the region.
Europe pediatric medical devices market is segmented into product, end user, and country. The pediatric medical devices market, by product, is segmented into in vitro diagnostic (IVD), cardiology devices, respiratory care, monitoring devices, neonatal ICU devices, and others. The in vitro diagnostic segment is likely to hold the largest share of the market in 2021. Based on end user, the pediatric medical devices market is segmented into hospitals, pediatric clinics, and others. The hospitals segment is likely to hold the largest share of the market in 2021. Based on country, the Europe pediatric medical devices market is segmented into France, Germany, Italy, Spain, UK, and rest of Europe. Germany held the largest market share in 2021.
A few major primary and secondary sources referred to for preparing this report on the pediatric medical devices market in Europe are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Atom Medical Corp.; F. Hoffmann-La Roche Ltd.; Fritz Stephan GmbH; General Electric Company; Hamilton Medical; Koninklijke Philips N.V.; Medtronic; Siemens AG; and TSE MEDICAL.
The Europe Pediatric Medical Devices Market is valued at US$ 8,253.86 Million in 2021, it is projected to reach US$ 13,862.68 Million by 2028.
As per our report Europe Pediatric Medical Devices Market, the market size is valued at US$ 8,253.86 Million in 2021, projecting it to reach US$ 13,862.68 Million by 2028. This translates to a CAGR of approximately 7.7% during the forecast period.
The Europe Pediatric Medical Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Pediatric Medical Devices Market report:
The Europe Pediatric Medical Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Pediatric Medical Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Pediatric Medical Devices Market value chain can benefit from the information contained in a comprehensive market report.