Nitinol refers to an alloy of nickel and titanium that is rapidly becoming a metal of choice for composition of various medical devices in the healthcare industry. Nitinol widely finds its applications as self-expanding grafts, baskets, filters, graft-supporting systems, and others. Nitinol alloys are most known for their super-elasticity and thermal shape memory.
Strategic insights for the Europe Nitinol Medical Devices provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
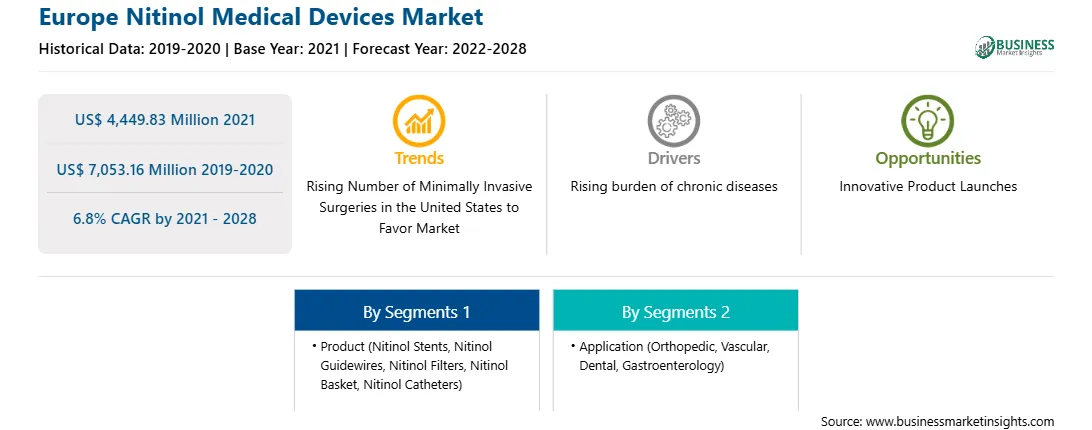
| Market size in 2021 | US$ 4,449.83 Million |
| Market Size by 2028 | US$ 7,053.16 Million |
| Global CAGR (2021 - 2028) | 6.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Nitinol Medical Devices refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
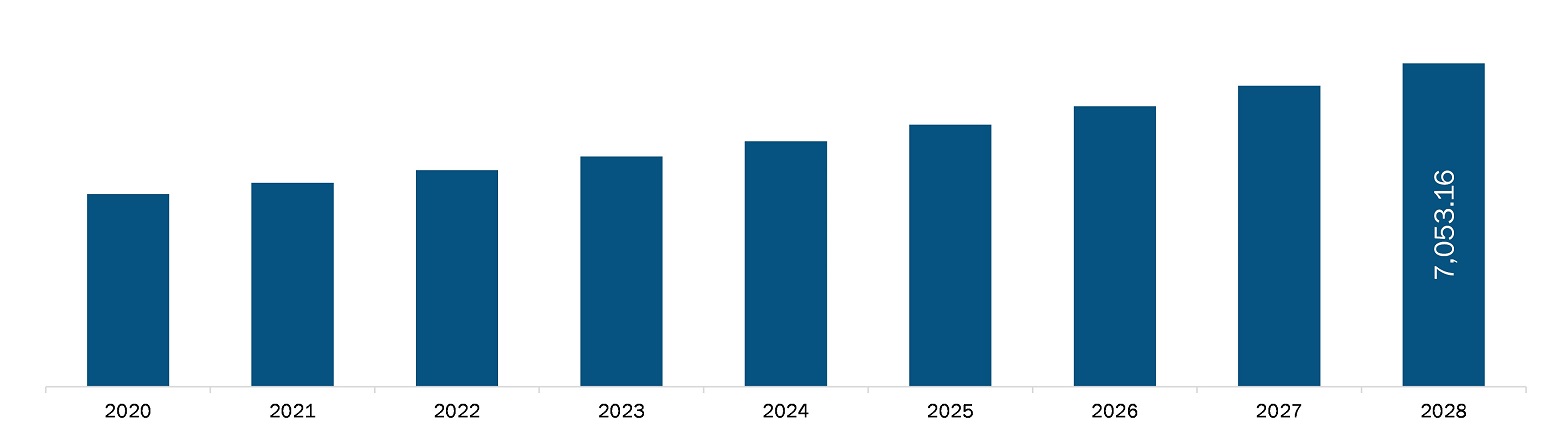
The Europe nitinol medical devices market is expected to reach US$ 7,053.16 million by 2028 from US$ 4,449.83 million in 2021; it is estimated to grow with a CAGR of 6.8% from 2021 to 2028. The growth of the market is attributed to increasing adoption of minimally invasive surgeries (MIS) and formation of regulatory guidelines on nitinol device biocompatibility by regulatory bodies are boosting the market. However, the manufacturing challenges including joining and assembly, plating and coating, machining, and stamping, and forming are likely to hinder the market’s growth.
The use of minimally invasive surgeries has evolved the paradigm of the surgery in the medical science. The advantages of MIS such as smaller incisions, reduced pain, lower complications, minimal blood loss and shorter hospital stays have dramatically influenced the adoption of MIS. The increasing adoption of MIS is majorly backed by advantages of the MIS has equally contributed to leveraging the growth of nitinol medical devices specifically for robotic surgeries and laparoscopic procedures. Hence, increasing number of procedures through MIS is likely to drive the market during the forecast period. However, owing to the several manufacturing challenges, regulatory bodies are taking efforts to enhance the biocompatibility and stability of nitinol devices for broader medical applications. Various evaluations are performed to build a uniform manufacturing guideline for nitinol-based products. In October 2020, regulatory bodies such as Food and Drug Administration (FDA) released new guidelines related to premarket submissions on nitinol device biocompatibility with skin and their technicalities. Thus, the release of guidelines is expected to enhance the use of nitinol for developing medical devices for broader applications, thereby providing vital growth opportunities to the manufacturers during the forecast period.
The COVID–19 pandemic has shown a significant negative impact on the European health economies. Health services in European region is highly prioritized to serve for patients affected by COVID-19 and routine health care services have remained suspended. Therefore, it has affected the nitinol medical devices related market and its dependable revenue generation in the region. Lockdown by European countries has widely affected the flow of businesses across the region and outside the region. Various conferences related to industry are being conducted through webinars. Hence, it is expected that negative impact on the nitinol medical devices market is likely to continues in the following few years.
The Europe nitinol medical devices market, by product, is segmented into nitinol stents, nitinol guidewires, nitinol filters, nitinol baskets, nitinol catheters and others. In 2020, the nitinol stents segment held the largest share of the market, by product. And the same segment is estimated to grow at significant CAGR during the forecast period.
The Europe nitinol medical devices market, by application, is segmented into orthopedic, vascular, dental and gastroenterology. In 2020, the vascular segment held the largest share of the market, by application. Also, similar segment is expected to grow at the fastest rate during the coming years.
A few of the primary and secondary sources associated with this report on the Europe nitinol medical devices market are the World Health Organization (WHO), Spanish National Health System (NHS), and Kidney Care UK.
By Product
By Application
The Europe Nitinol Medical Devices Market is valued at US$ 4,449.83 Million in 2021, it is projected to reach US$ 7,053.16 Million by 2028.
As per our report Europe Nitinol Medical Devices Market, the market size is valued at US$ 4,449.83 Million in 2021, projecting it to reach US$ 7,053.16 Million by 2028. This translates to a CAGR of approximately 6.8% during the forecast period.
The Europe Nitinol Medical Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Nitinol Medical Devices Market report:
The Europe Nitinol Medical Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Nitinol Medical Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Nitinol Medical Devices Market value chain can benefit from the information contained in a comprehensive market report.