Europe Monoclonal Antibodies Market
No. of Pages: 172 | Report Code: TIPRE00027983 | Category: Life Sciences
No. of Pages: 172 | Report Code: TIPRE00027983 | Category: Life Sciences
Today, mAB treatment for people suffering from COVID-19 approved by the FDA intended for emergency use reveals that it proves successful in reducing the chances of severe diseases, hospitalization, and death by almost 70% by shortening hospital stays. Apart from that, coverage of mAB products for treating COVID-19 is another contributing factor for the growth of this market during the onset of the pandemic. Furthermore, the National Institute for Health and Care Excellence (NICE) updates managing COVID-19 guidelines with monoclonal antibody recommendations. For records, in October 2021, the new advice recommended offering a combination of "casirivimab" and "imdevimab" for treating COVID-19 patients aged 12 and above who are in hospital. Moreover, both the products are licensed for the prophylaxis and treatment of acute COVID-19 infection.
The European market is severely hit due to the exponential increase of COVID-19 cases in the region. Many nations are revealing more patients per day now than during the first wave earlier this year. Lockdowns are being reintroduced in UK, Spain, and Italy, and Ireland, as per the European Centre for Disease Prevention and Control (ECDC). Due to the second wave of COVID-19 crises, the government of many nations is heading towards mounting up testing capacity. Countries in the European regions were profoundly affected due to the COVID-19 pandemic. For instance, countries such as Italy, Spain, and France have recorded the most significant number of positive cases and have registered the maximum number of deaths. The rising rate of coronavirus results in increased stress on the region's healthcare system, raising the demand for diagnostic tests in its healthcare system, supporting the expansion of the sector in this region. Moreover, In December 2021, Pfizer and BioNTech announced that the companies will amend the clinical safety study while evaluating the safety, tolerability, and immunogenicity of the Pfizer-BioNTech COVID-19 vaccine in children 6 months to under 5 years of age.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the monoclonal antibodies market. The Europe monoclonal antibodies market is expected to grow at a good CAGR during the forecast period.
Europe Monoclonal Antibodies Market Segmentation
Companies Mentioned
Strategic insights for the Europe Monoclonal Antibodies provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
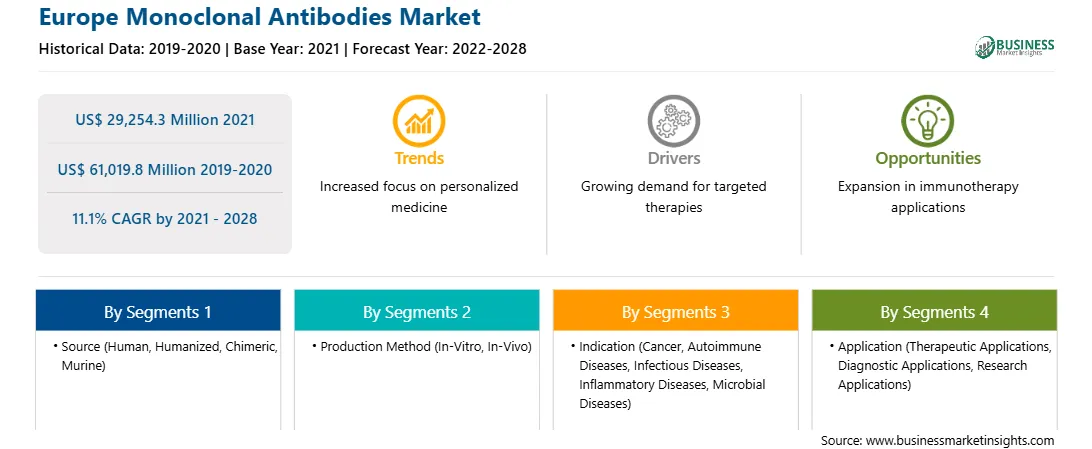
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 29,254.3 Million |
| Market Size by 2028 | US$ 61,019.8 Million |
| Global CAGR (2021 - 2028) | 11.1% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Source
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Monoclonal Antibodies refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
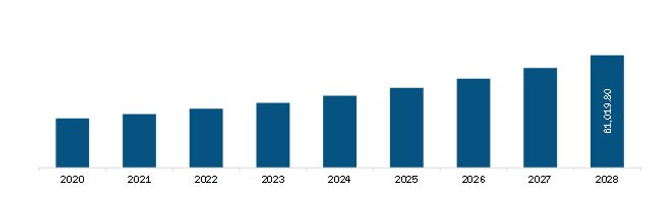
The Europe Monoclonal Antibodies Market is valued at US$ 29,254.3 Million in 2021, it is projected to reach US$ 61,019.8 Million by 2028.
As per our report Europe Monoclonal Antibodies Market, the market size is valued at US$ 29,254.3 Million in 2021, projecting it to reach US$ 61,019.8 Million by 2028. This translates to a CAGR of approximately 11.1% during the forecast period.
The Europe Monoclonal Antibodies Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Monoclonal Antibodies Market report:
The Europe Monoclonal Antibodies Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Monoclonal Antibodies Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Monoclonal Antibodies Market value chain can benefit from the information contained in a comprehensive market report.