Europe Medical Device Vigilance Software Market
No. of Pages: 101 | Report Code: BMIRE00030961 | Category: Technology, Media and Telecommunications
No. of Pages: 101 | Report Code: BMIRE00030961 | Category: Technology, Media and Telecommunications
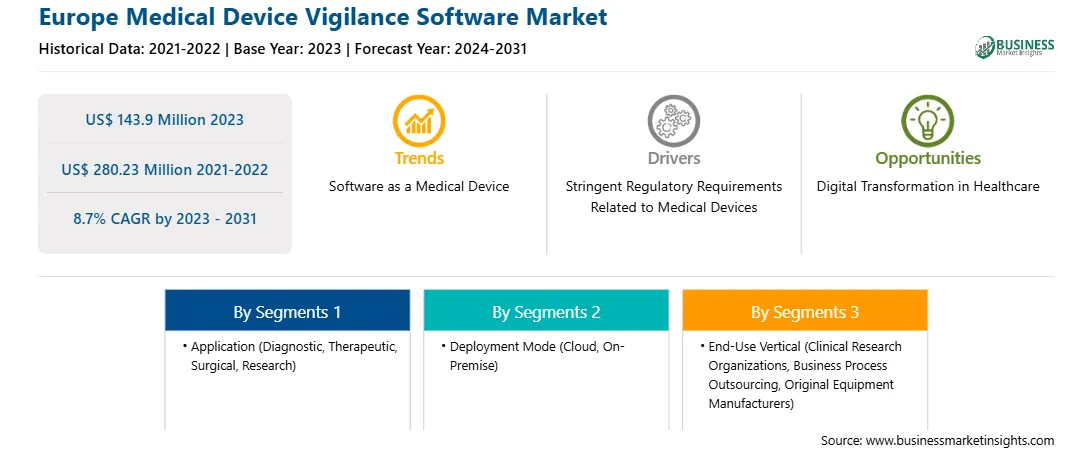
The Europe Medical Device Vigilance Software market size was valued at US$ 143.9 million in 2023 and is expected to reach US$ 280.23 million by 2031; it is estimated to record a CAGR of 8.7% from 2023 to 2031.
The Europe medical device vigilance software market consists of countries such as Germany, France, the UK, Spain, Russia, and the Rest of Europe. Germany dominated the market in 2023, followed by France and UK respectively. In terms of revenue, Germany held significant the Medical Device Vigilance Software market share in 2023. Owing to the high rate of technology adoption, coupled with the presence of various multinational companies, Germany serves as a significant expansion base for the manufacturing sector in Europe. Germany's energy consumption is significant, making it the largest energy consumer in the region, surpassing France by 40%. The country's consumption levels are driven by its extensive industrial and economic activities and its massive population. In addition, a rise in investment toward energy transition by investing in heating and cooling systems such as Medical Device Vigilance Software are expected to boost the market in the coming years. However, the impacts of the COVID-19 outbreak are still impacting production in the manufacturing industry. The production dropped by 1.2% in 2023 as compared to 2022. Four key industries driving the economic growth of the country include automotive, mechanical engineering, chemical, and electrical industries. BASF SE, Siemens AG, Daimler AG, and other major players have established manufacturing facilities in the country. These companies are planning to expand their facilities to boost production as well as fulfill consumer demand across the country. Therefore, the expansion of these industries is anticipated to fuel the medical device vigilance software market growth in the coming years in Germany.
Based on End Use Vertical, the market is divided into CROs, BPO, OEMs, and others. The original equipment manufacturers segment held the largest medical device vigilance software market share in 2023. Medical device original equipment manufacturers (OEMs) are responsible for the entire lifecycle of the medical devices which includes its engineering, production, and often compliance with regulatory standards. In addition, after the medical device is sold, OEMs are responsible for the post-market surveillance of their medical devices to monitor their performance and safety in the market. OEMs are the primary end users of medical device vigilance software. It helps them ensure the safety of their products throughout the product lifecycle, including post-market surveillance, adverse event reporting, and compliance with regulations. The software helps in real-time tracking of their product performance. It also allows them to identify and respond quickly to any safety signals and ensure that issues are addressed promptly. The medical device vigilance software provided in Europe assists the OEMs with European medical device vigilance reporting, post-market surveillance, and creating procedures for such activities. They also help the OEMs stay compliant with Medical Device Regulation (MDR) No. 2017/745 (for medical devices or active implantable medical devices (MDs)) and In Vitro Diagnostic Device Regulation (IVDR) No. 2017/746 (for in vitro diagnostic (IVD) devices)..
Oracle Corp; AB Cube S.A.S.; Sarjen Systems Pvt. Ltd; AssurX, Inc.; UL Solutions Inc; Honeywell International Inc; PTC Inc; Intel Corp; Max Application; and Xybion Digital Inc. are among the key Medical Device Vigilance Software market players that are profiled in this market study.
The overall Europe Medical Device Vigilance Software market size has been derived using both primary and secondary sources. Exhaustive secondary research has been conducted using internal and external sources to obtain qualitative and quantitative information related to the Europe Medical Device Vigilance Software size. The process also helps obtain an overview and forecast of the market with respect to all the market segments. Also, multiple primary interviews have been conducted with industry participants to validate the data and gain analytical insights. This process includes industry experts such as VPs, business development managers, market intelligence managers, and national sales managers, along with external consultants such as valuation experts, research analysts, and key opinion leaders, specializing in the Medical Device Vigilance Software market.
Strategic insights for the Europe Medical Device Vigilance Software provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 143.9 Million |
| Market Size by 2031 | US$ 280.23 Million |
| Global CAGR (2023 - 2031) | 8.7% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Application
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Medical Device Vigilance Software refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Europe Medical Device Vigilance Software Market is valued at US$ 143.9 Million in 2023, it is projected to reach US$ 280.23 Million by 2031.
As per our report Europe Medical Device Vigilance Software Market, the market size is valued at US$ 143.9 Million in 2023, projecting it to reach US$ 280.23 Million by 2031. This translates to a CAGR of approximately 8.7% during the forecast period.
The Europe Medical Device Vigilance Software Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Medical Device Vigilance Software Market report:
The Europe Medical Device Vigilance Software Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Medical Device Vigilance Software Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Medical Device Vigilance Software Market value chain can benefit from the information contained in a comprehensive market report.