Europe Manufacturing Execution System (MES) In Life Sciences Market
No. of Pages: 145 | Report Code: BMIRE00028413 | Category: Technology, Media and Telecommunications
No. of Pages: 145 | Report Code: BMIRE00028413 | Category: Technology, Media and Telecommunications
Pharmaceutical and medical device manufacturers face various challenges in a drastically changing landscape. Protectionist trade policies are creating supply chain challenges while increased state interventions are promoting regionalization. High inflation across several countries is increasing labor, raw materials, and transportation costs. The pharma industry is also facing talent shortages linked to broader labor market trends, including the 20% increase in demand for science, technology, engineering, and mathematics (STEM) related roles across the life sciences industry. The product landscape is also changing rapidly, with new modalities such as cell and gene therapy (CGT) and mRNA vaccine technology witnessing a high percent rise in the drug development pipeline. Despite being renowned for holding high inventory levels and long-standing dual-sourcing policies, the changing landscape has led pharmaceutical and medical device manufacturers to optimize their business processes, including adopting MES. Previously used paper-based systems are becoming obsolete as a larger emphasis is being laid upon gathering real-time data for improving process flow and reducing operational costs. MES can help manufacturers to function with a lower workforce, gather accurate real-time data from the production process, increase process efficiency and flexibility, safeguard regulatory expectations, and digitize the manufacturing process.
For instance, in September 2020, Korber and Walvax Biotechnology announced that it had signed an agreement to implement Werum PAS-X MES, the MES for pharma, biotech, and cell & gene therapy manufacturing, at its Yuxi plant for vaccine production. As per Korber, PAS-X MES allows the user to manage, visualize and analyze data fast, comprehensively, and in real time. Because of all the above advantages of using MES, various market players are adopting the system.
The Europe MES in life sciences market is segmented into Germany, the UK, France, Italy, Spain, and the Rest of Europe. Europe houses a few of the major medical device manufacturers, and the medical device industry in the region has experienced several cases of MES deployment in the past few years. As medical device companies are embracing digitalization in areas ranging from factory floor automation to back-office administration providing solutions to enhance efficiency in all departments. In May 2018, B Braun, one of the major medical device manufacturers headquartered in Europe, adopted the MES technology in its Melsungen facility. This deployment enabled B. Braun to adopt paperless digital manufacturing processes, thereby improving flexibility, agility, operational effectiveness, and product quality while cutting costs. Later, the company successfully updated its existing PAS-X system from PAS-X V2 to PAS-X V3 in plant A, headquartered in Melsungen. With MES, medical device companies can automatically control their manufacturing environment and easily manage regulatory inspections, alongside keeping up with the changing FDA and EU MRD requirements in a long run. AstraZeneca, Fareva, Ipsen, and Sanofi S.A. are a few of the pharmaceutical companies headquartered in Europe, which have implemented the XFP MES Suite offered by Elan, which is a part of Siemens. This suite provides production visibility and control throughout the enterprise by optimizing the entire production cycle, from raw material delivery to end product shipment, in compliance with 21CFR part 11 and ISA 88/95.
Strategic insights for the Europe Manufacturing Execution System (MES) In Life Sciences provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Europe Manufacturing Execution System (MES) In Life Sciences refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
Europe Manufacturing Execution System (MES) In Life Sciences Strategic Insights
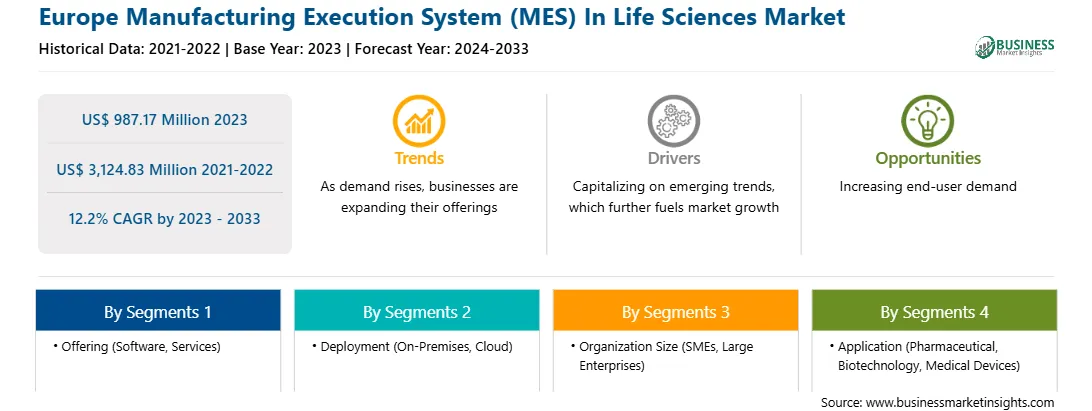
Europe Manufacturing Execution System (MES) In Life Sciences Report Scope
Report Attribute
Details
Market size in 2023
US$ 987.17 Million
Market Size by 2033
US$ 3,124.83 Million
Global CAGR (2023 - 2033)
12.2%
Historical Data
2021-2022
Forecast period
2024-2033
Segments Covered
By Offering
By Deployment
By Organization Size
By Application
Regions and Countries Covered
Europe
Market leaders and key company profiles
Europe Manufacturing Execution System (MES) In Life Sciences Regional Insights
The Europe manufacturing execution system in life sciences market is segmented into offering, deployment, organization size, application, and country.
Based on offering, the Europe manufacturing execution system in life sciences market is segmented into software and services. The services segment held a larger share of the Europe manufacturing execution system in life sciences market in 2023.
Based on deployment, the Europe manufacturing execution system in life sciences market is segmented into on-premises and cloud. The cloud segment held a larger share of the Europe manufacturing execution system in life sciences market in 2023.
Based on organization size, the Europe manufacturing execution system in life sciences market is segmented into SMEs and large enterprises. The large enterprises segment held the largest share of the Europe manufacturing execution system in life sciences market in 2023.
Based on application, the Europe manufacturing execution system in life sciences market is segmented into pharmaceutical, biotechnology, and medical devices. The medical devices segment held the largest share of the Europe manufacturing execution system in life sciences market in 2023.
Based on country, the Europe manufacturing execution system in life sciences market is segmented into Germany, France, Italy, the UK, Spain, and the Rest of Europe. Germany dominated the share of the Europe manufacturing execution system in life sciences market in 2023.
ATS Global B.V.; Emerson Electric Co; Korber AG; LZ Lifescience Limited; Rockwell Automation Inc; Schneider Electric SE; and Siemens AG are the leading companies operating in the Europe manufacturing execution system in life sciences market.
The Europe Manufacturing Execution System (MES) In Life Sciences Market is valued at US$ 987.17 Million in 2023, it is projected to reach US$ 3,124.83 Million by 2033.
As per our report Europe Manufacturing Execution System (MES) In Life Sciences Market, the market size is valued at US$ 987.17 Million in 2023, projecting it to reach US$ 3,124.83 Million by 2033. This translates to a CAGR of approximately 12.2% during the forecast period.
The Europe Manufacturing Execution System (MES) In Life Sciences Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Manufacturing Execution System (MES) In Life Sciences Market report:
The Europe Manufacturing Execution System (MES) In Life Sciences Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Manufacturing Execution System (MES) In Life Sciences Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Manufacturing Execution System (MES) In Life Sciences Market value chain can benefit from the information contained in a comprehensive market report.