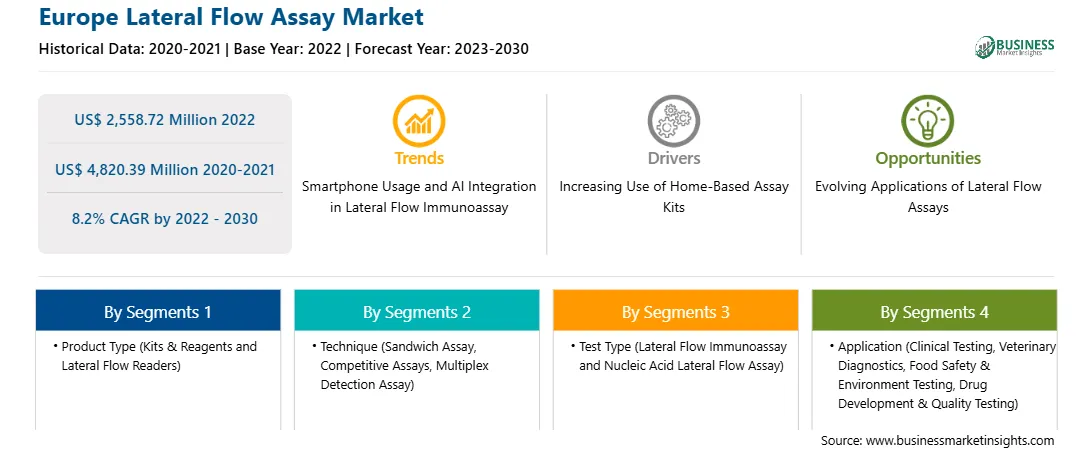
The Europe lateral flow assay market was valued at US$ 2,558.72 million in 2022 and is expected to reach US$ 4,820.39 million by 2030; it is estimated to register a CAGR of 8.2% from 2022 to 2030.
Lateral flow assay-based point-of-care (POC) diagnostic tools are rapid, easy-to-use, and low-cost paper-based procedures, which are specifically perceived to be beneficial in resource-limited settings and industrialized countries. These tests are increasingly replacing prolonged, conventional laboratory methods. No training and complex infrastructures are required to run POC diagnostic tests. Thus, these tests cost less than conventional laboratory diagnostic techniques. POC testing tools are expected to be a crucial revenue pocket in the lateral flow assay market because of their prominent role in combating the burgeoning disease burden. Many companies are engaged in developing innovative lateral flow assay-based POC devices for infectious disease diagnosis, drugs-of-abuse screening, pregnancy (using hCG levels) and ovulation confirmation, and blood protein marker measurement.
The rising popularity of POC testing and ongoing developments in lateral flow assay-based POC testing fuel the growth of the lateral flow assay market.
Germany, the UK, France, Italy, and Spain are the major countries in Europe, including the rest of European countries. Germany held the largest market for lateral flow assay in the region in 2022. The growth of the lateral flow assay market in the region is attributed to factors such as the increasing geriatric population, the growing rate of accidents, and population growth in the region. Other major drivers include technological innovations and increasing demand for technologies in Germany, France, and the UK.
The pharmaceutical industry in Germany holds the global fourth largest position with small and mid-sized companies. According to the International Trade Administration (ITA), the pharmaceutical market in Germany accounted for US$ 62.5 billion in 2019 and will expect to increase in future. The country is likely to remain one of the most attractive destinations for the global pharmaceutical industry in the coming years in terms of manufacturing and supply. According to the ECDC's Annual epidemiological report, chlamydia infection, campylobacteriosis, salmonellosis, gonorrhea, and tuberculosis were the most frequently reported notifiable infectious diseases in the EU and The European Economic Area (EEA).
Strategic insights for the Europe Lateral Flow Assay provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 2,558.72 Million |
| Market Size by 2030 | US$ 4,820.39 Million |
| Global CAGR (2022 - 2030) | 8.2% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Lateral Flow Assay refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Europe lateral flow assay market is categorized into product type, technique, test type, application, end user, and country.
Based on product type, the Europe lateral flow assay market is bifurcated into kits & reagents and lateral flow readers. The kits & reagents segment held a larger Europe lateral flow assay market share in 2022.
In terms of technique, the Europe lateral flow assay market is segmented into sandwich assay, competitive assays, and multiplex detection assay. The sandwich assay segment held the largest Europe lateral flow assay market share in 2022.
By test type, the Europe lateral flow assay market is divided into lateral flow immunoassay and nucleic acid lateral flow assay. The lateral flow immunoassay segment held a larger Europe lateral flow assay market share in 2022.
Based on application, the Europe lateral flow assay market is categorized into clinical testing, veterinary diagnostics, food safety & environment testing, and drug development & quality testing. The clinical testing segment held the largest Europe lateral flow assay market share in 2022.
By end user, the Europe lateral flow assay market is segmented into hospitals and clinics, diagnostics laboratories, homecare, veterinary clinics, pharmaceutical & biotechnology companies, and others. The hospitals and clinics segment held the largest Europe lateral flow assay market share in 2022.
By country, the Europe lateral flow assay market is segmented into the UK, Germany, France, Spain, Italy, Benelux, and the Rest of Europe. Germany dominated the Europe lateral flow assay market share in 2022.
F. Hoffmann-La Roche Ltd, Siemens Healthineers AG, Becton Dickinson and Co, PerkinElmer Inc, Hologic Inc, QIAGEN NV, bioMerieux SA, QuidelOrtho Corp, Abbott Laboratories, Merck KGaA, Bio-Rad Laboratories Inc, and Thermo Fisher Scientific Inc are some of the leading companies operating in the Europe lateral flow assay market.
1. Abbott Laboratories
2. Becton Dickinson and Co
3. bioMerieux SA
4. Bio-Rad Laboratories Inc
5. F. Hoffmann-La Roche Ltd
6. Hologic Inc
7. Merck KGaA
8. PerkinElmer Inc
9. QIAGEN NV
10. QuidelOrtho Corp
11. Siemens Healthineers AG
12. Thermo Fisher Scientific Inc
The Europe Lateral Flow Assay Market is valued at US$ 2,558.72 Million in 2022, it is projected to reach US$ 4,820.39 Million by 2030.
As per our report Europe Lateral Flow Assay Market, the market size is valued at US$ 2,558.72 Million in 2022, projecting it to reach US$ 4,820.39 Million by 2030. This translates to a CAGR of approximately 8.2% during the forecast period.
The Europe Lateral Flow Assay Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Lateral Flow Assay Market report:
The Europe Lateral Flow Assay Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Lateral Flow Assay Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Lateral Flow Assay Market value chain can benefit from the information contained in a comprehensive market report.