Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market
No. of Pages: 129 | Report Code: BMIRE00027543 | Category: Life Sciences
No. of Pages: 129 | Report Code: BMIRE00027543 | Category: Life Sciences
Awareness Among Individuals Regarding Diagnosis of H. pylori Fuel Europe Helicobacter Pylori (H. pylori)
Diagnosis of H. pylori infection is performed by invasive (endoscopy and endoscopic biopsy for histopathology, culture, and rapid urease test) and non-invasive (urea breath tests, stool antigen test, and serological tests) methods. The diagnostic decisions are based on the prevalence of H. pylori infection and the number of incidences of age-related gastric cancer. For instance, non-invasive techniques are preferred mostly when the incidence of gastric cancer is low. However, endoscopy is recommended for patients who have a high probability of being diagnosed with gastric cancer, such as patients above 60 years of age or younger patients in a few European countries, or patients with a family history of gastric cancer, or countries with a high incidence of gastric cancer. Further, precise accuracy is obtained by using multiple diagnostic tests. Although these tests have high accuracy, endoscopy-based diagnostic methods are not recommended for screening purposes, mainly due to their invasiveness, high cost, and unavailability. However, in a few European countries, endoscopy is recommended for patients over the age of 45 years who have a high chance of developing gastric cancer. Thus, governments of various European countries are boosting awareness of the diagnosis of H. pylori, which is expected to fuel the market growth.
Further, in 2020, RedHill Biopharma launched a nationwide H. pylori disease state educational field led by its sales team. The launch is intended to provide greater awareness to healthcare professionals regarding the risk of H. pylori infection and the growing resistance of H. pylori to standard care of antibiotics, which leads to 25-40% failure of current therapies.
Market Overview
As per the Elsevier B.V. report, H. pylori infects ~25% of the German population and causes gastritis that becomes more complicated with peptic ulcer disease, gastric adenocarcinoma, or mucosa-associated lymphoid tissue (MALT) lymphoma annually. According to the National Institute of Health (NIH) report, H. pylori infection causes chronic inflammation that significantly increases the risk of developing duodenal and gastric ulcer disease and gastric cancer. Additionally, H. pylori infection is the strongest known risk factor for gastric cancer and is the second-leading cause of cancer-related deaths globally. As per the same report, ~15,500 people annually have gastric cancer, with a decreasing number of incidences and a 5-year survival rate of 30-35%. Moreover, Germany has no gastric cancer prevention programs, resulting in the disease being diagnosed frequently in locally advanced stages. Such aforementioned factors are responsible for the growth of the Helicobacter pylori (H. pylori) non-invasive testing market.
Strategic insights for the Europe Helicobacter Pylori (H. pylori) Non-invasive Testing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Europe Helicobacter Pylori (H. pylori) Non-invasive Testing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Strategic Insights
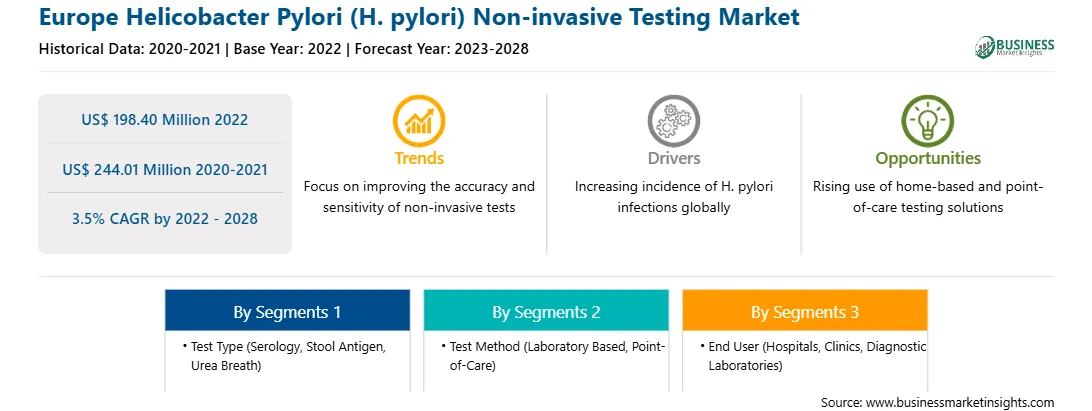
Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Report Scope
Report Attribute
Details
Market size in 2022
US$ 198.40 Million
Market Size by 2028
US$ 244.01 Million
Global CAGR (2022 - 2028)
3.5%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Test Type
By Test Method
By End User
Regions and Countries Covered
Europe
Market leaders and key company profiles
Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Regional Insights
Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market Segmentation
The Europe helicobacter pylori (H. pylori) non-invasive testing market is segmented on test type, test method, end user, and country.
Based on test type, the Europe Helicobacter pylori (H. pylori) non-invasive testing market is segmented into serology, stool antigen, and urea breathe. In 2022, the urea breath segment is expected to hold the largest share of the market. Based on test method, the Europe Helicobacter pylori (H. pylori) non-invasive testing market is bifurcated into laboratory based and point of care. The laboratory based segment is expected to hold a larger share of the market in 2022. Based on end user, the Europe Helicobacter pylori (H. pylori) non-invasive testing market is segmented into hospitals, clinics, and diagnostic laboratories. The diagnostic laboratories segment is expected to hold the largest share of the market in 2022. Based on country, the market is segmented into France, Germany, Italy, the UK, Spain, and the Rest of Europe. Further, Germany dominated the market in 2022. Abbott Laboratories; Bio-Rad Laboratories Inc.; CerTest Biotec; Coris BioConcept; DiaSorin S.p.A.; Meridian Bioscience Inc.; QuidelOrtho Corporation; Sekisui Diagnostics; Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd; and Thermo Fisher Scientific Inc. are the leading companies operating in the helicobacter pylori (H. pylori) non-invasive testing market in Europe.
The Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market is valued at US$ 198.40 Million in 2022, it is projected to reach US$ 244.01 Million by 2028.
As per our report Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market, the market size is valued at US$ 198.40 Million in 2022, projecting it to reach US$ 244.01 Million by 2028. This translates to a CAGR of approximately 3.5% during the forecast period.
The Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market report:
The Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Helicobacter Pylori (H. pylori) Non-invasive Testing Market value chain can benefit from the information contained in a comprehensive market report.