The Europe healthcare regulatory affairs outsourcing market includes the consolidated markets for Germany, France, Italy, Spain, United Kingdom, and Rest of Europe. An increase in geographical expansion activities by companies that aim for speedy approvals in local markets is expected is likely to be the major factor driving the growth of this market in the European region. However, loss of control and technological advances leading to fluctuations in pricing are expected to hamper the market growth in the forecasting period. Changes in the reimbursement scenario and pricing pressure are expected to increase the adoption of cost-saving measures by the pharmaceutical and medical device companies in Germany. This is anticipated to promote off-shoring of regulatory affairs outsourcing. Pharmaceutical companies are outsourcing regulatory affairs services to get high-quality documents, control the R&D cost, and reduce investment costs required to train the regulatory affairs team. Furthermore, the pharmaceutical and medical device companies are outsourcing their regulatory affairs services to diverge their business activities and manage product life cycles. Rise in demand for speedy approval of new products is the major factor driving the growth of the Europe healthcare regulatory affairs outsourcing market.
Europe has experienced an exponential increase in COVID-19 cases. Many countries in the region recorded a large number of patients per day during the first wave. With onset of the second wave, bans on social gatherings were reintroduced in the second half of 2020, in the UK, Spain, Italy, and Ireland, according to the European Center for Disease Prevention and Control (ECDC). Government have been prioritizing the improvement of test capacities. The exponentially rising infection rate is putting high burden on the region's healthcare system, increasing the demand for diagnostic tests in their healthcare system and supporting the expansion of the sector in this region. The COVID-19 pandemic has led to delays in research and development, manufacturing, supply chain, and almost all components critical to drug development. Communication with regulators has become unpredictable due to the long wait times for feedback from review teams or agencies critical to the development process. In addition, timeline crunches and around-the-clock staff availability are particular challenges faced by market participants. However, a large number of ongoing clinical trials in response to the COVID-19 pandemic are contributing to the growth of the clinical trial service segment. The urgency to identify and commercialize an effective cure and vaccine for COVID-19 disease has increased the number of clinical trials across Europe. In addition, supportive regulatory measures for shortening trial approval times, waiving the waiting periods, publishing guidance documents, and regulatory funding for clinical trials are expected to further fuel segment growth.

Strategic insights for the Europe Healthcare Regulatory Affairs Outsourcing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
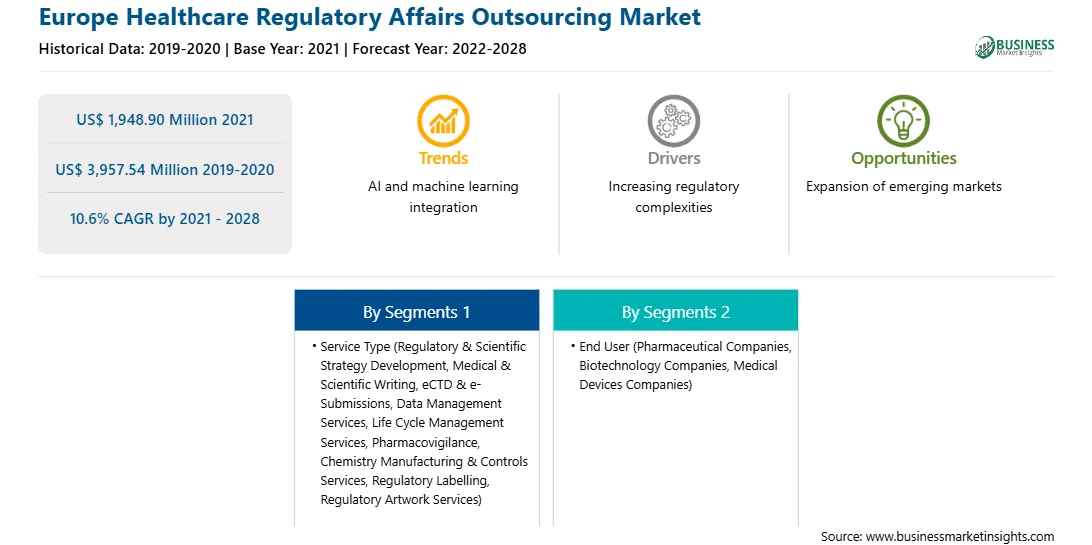
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 1,948.90 Million |
| Market Size by 2028 | US$ 3,957.54 Million |
| Global CAGR (2021 - 2028) | 10.6% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Healthcare Regulatory Affairs Outsourcing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The healthcare regulatory affairs outsourcing market in Europe is expected to grow from US$ 1,948.90 million in 2021 to US$ 3,957.54 million by 2028; it is estimated to grow at a CAGR of 10.6% from 2021 to 2028. Continuous upgrades and progress in traditional drug development approaches are creating significant challenges in the healthcare sector. There is tremendous pressure on the pharmaceutical companies and medical fraternity to reduce the cost of prescription drugs, while their operational costs are skyrocketing. The complexity of regulatory requirements, declining revenues due to blockbuster drugs going off patent, and pressure from governments as well as health insurers for reduction in healthcare cost has presented additional challenges to healthcare industries. Given these difficulties, pharmaceutical companies have realized the need to leverage their resources along with the expertise provided by specialist external sources. Many high-end regulatory consulting companies are offering their expertise across the complete product life cycle. The outsourcing of regulatory affairs may enable sponsors to gain experience, optimize cost, and enhance productivity. Regulatory outsourcing companies are in better position to assess regulatory requirements, which allows them to select the best solutions. They are well versed with understanding associated with implementing, operating, and maintaining a regulatory publishing system. Most of the big pharmaceutical and biotechnology companies look out for consulting companies that can also offer supporting regulatory and pharmacovigilance services. The increased complexity of regulatory filings underlines the demand for specialist CRO expertise. Having planned product-specific regulatory advice and strategies, along with healthcare regulatory compliance measures, in an early stages of product development is extremely important for the regulatory approval of the products. Failure to address the compliance in the early stage of development often leads to delay in the approval process due to inappropriately filed documentations, manufacturing oversights, omitted regulatory studies, and other failures to meet the regulatory requirements. Healthcare companies are now focusing on their core competencies and outsourcing the noncore functions to improve productivity and operational efficiency. Therefore, increasing regulatory pressure on healthcare companies is expected to drive the market growth.
The Europe healthcare regulatory affairs outsourcing market has been segmented based on service type, end user, and country. On the basis of service type, the Europe healthcare regulatory affairs outsourcing market is segmented into medical & scientific writing, pharmacovigilance, data management services, life cycle management services, eCTD and e-Submissions, regulatory and scientific strategy development, chemistry manufacturing and controls (CMC) services, regulatory labelling, and regulatory artwork services. The medical & scientific writing segment dominated the market in 2020 and pharmacovigilance segment is expected to be the fastest growing during the forecast period. Based on end user, the market is segmented into pharmaceutical companies, biotechnology companies, and medical devices companies. The pharmaceutical companies segment dominated the market in 2020 and is expected to be the fastest growing during the forecast period. Likewise, the medical devices companies segmented is categorized into medical device materials & biomaterials, medical device, biomarkers and in vitro diagnostics (IVD), medical device software (SaMD), medical device electromechanics, medical device substance-based, and medical device of combination product.
A few major primary and secondary sources referred to for preparing this report on healthcare regulatory affairs outsourcing market in Europe are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Arriello Ireland Ltd., Asphalion S.L, Azierta Contract Science Support Consulting, DRA CONSULTING OY, IQVIA Inc., KLIFO, PAREXEL INTERNATIONAL CORPORATION, PHARMALEX GMBH, ProductLife Group, ProPharma Group, and Voisin Consulting Life Sciences (VCLS) are among others.
The Europe Healthcare Regulatory Affairs Outsourcing Market is valued at US$ 1,948.90 Million in 2021, it is projected to reach US$ 3,957.54 Million by 2028.
As per our report Europe Healthcare Regulatory Affairs Outsourcing Market, the market size is valued at US$ 1,948.90 Million in 2021, projecting it to reach US$ 3,957.54 Million by 2028. This translates to a CAGR of approximately 10.6% during the forecast period.
The Europe Healthcare Regulatory Affairs Outsourcing Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Healthcare Regulatory Affairs Outsourcing Market report:
The Europe Healthcare Regulatory Affairs Outsourcing Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Healthcare Regulatory Affairs Outsourcing Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Healthcare Regulatory Affairs Outsourcing Market value chain can benefit from the information contained in a comprehensive market report.