Europe Electronic Patient-Reported Outcomes (ePROS) Market
No. of Pages: 91 | Report Code: BMIRE00031352 | Category: Technology, Media and Telecommunications
No. of Pages: 91 | Report Code: BMIRE00031352 | Category: Technology, Media and Telecommunications
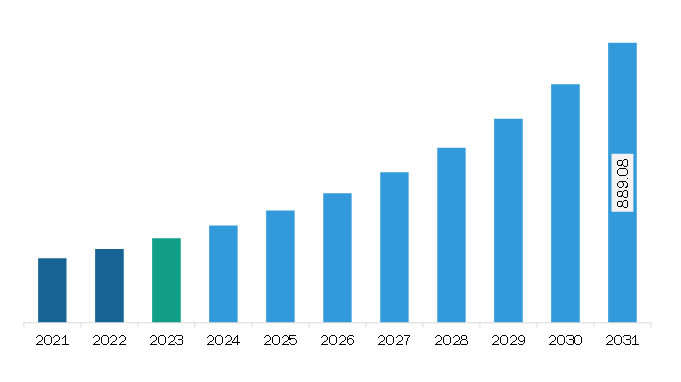
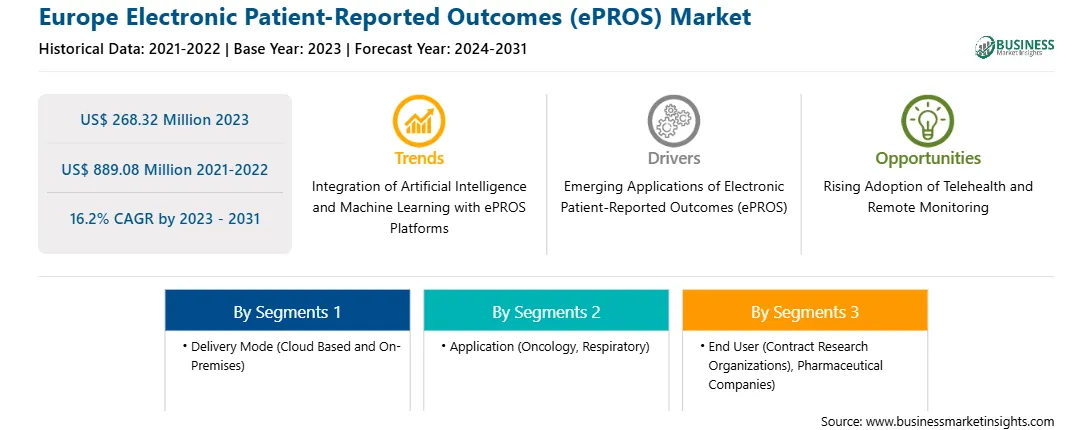
The Europe electronic patient-reported outcomes (ePROS) market was valued at US$ 268.32 million in 2023 and is expected to reach US$ 889.08 million by 2031; it is estimated to register a CAGR of 16.2% from 2023 to 2031.
Increasing Focus on Patient-Centered Care Drives Europe Electronic Patient-Reported Outcomes (ePROS) Market
The growing emphasis on patient-centered care fuels the growth of the electronic patient-reported outcomes (ePROS) market. Tools that gather patient feedback in real-time are in high demand owing to the shift toward prioritizing patient experiences, outcomes, and preferences. The real-time tracking of patient-reported health outcomes is made possible by ePROs as healthcare becomes more personalized and value-based. Better health outcomes are supported by ensuring treatments are tailored to each patient's needs. The use of digital tools such as ePROs has increased as the focus of healthcare facilities shifts to patient experience and satisfaction. With the help of this technology, patients can have less stress and receive continuous feedback by giving healthcare providers access to precise data. This change drives innovation in digital health technologies, supporting the integration of ePROs into medical procedures. For instance, healthcare providers can easily monitor patient outcomes owing to the growing integration of ePRO tools with electronic health record (EHR) systems. This facilitates the alignment of clinical decisions with the preferences and experiences that the patient has reported. Therefore, a greater understanding of patient-centered care propels the use of ePROs.
Europe Electronic Patient-Reported Outcomes (ePROS) Market Overview
According to Clinical Trials Arena, Germany held ~3.9% of the total clinical trials carried out worldwide in 2021. Furthermore, there is a massive patient pool and a high demand for quality healthcare in Germany. Coordinating centers for clinical trials were set up as part of a new funding program under the Federal Ministry of Education and Research to further encourage academic clinical research. The Federal Institute for Drugs and Medical Devices or the Paul-Ehrlich Institute approves clinical trials in Germany, depending on the product to be investigated. Thus, Germany's clinical trial approval processes are standardized, transparent, reliable, and approved for relatively short study startup timelines. To further enhance the clinical trial process and evaluation, the increased use of digital technologies such as ePROs plays an important role. In addition, the development of digital health technology and integration of artificial intelligence is also rising in the country. According to the Germany Trade and Invest report, the healthcare industry in Germany is undergoing massive digitalization. By 2025, the digital health market in Germany is projected to be worth US$ 63 billion. Therefore, the rise in clinical trials and an upsurge in the adoption of digital health technologies are expected to fuel the electronic related-patient outcomes (ePROS) market in the coming years.
Europe Electronic Patient-Reported Outcomes (ePROS) Market Revenue and Forecast to 2031 (US$ Million)
Strategic insights for the Europe Electronic Patient-Reported Outcomes (ePROS) provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Europe Electronic Patient-Reported Outcomes (ePROS) refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
Europe Electronic Patient-Reported Outcomes (ePROS) Strategic Insights
Europe Electronic Patient-Reported Outcomes (ePROS) Report Scope
Report Attribute
Details
Market size in 2023
US$ 268.32 Million
Market Size by 2031
US$ 889.08 Million
Global CAGR (2023 - 2031)
16.2%
Historical Data
2021-2022
Forecast period
2024-2031
Segments Covered
By Delivery Mode
By Application
By End User
Regions and Countries Covered
Europe
Market leaders and key company profiles
Europe Electronic Patient-Reported Outcomes (ePROS) Regional Insights
Europe Electronic Patient-Reported Outcomes (ePROS) Market Segmentation
The Europe electronic patient-reported outcomes (ePROS) market is categorized into delivery mode, application, end user, and country.
By delivery mode, the Europe electronic patient-reported outcomes (ePROS) market is bifurcated into cloud based and on-premises. The cloud based segment held a larger share of the Europe electronic patient-reported outcomes (ePROS) market share in 2023.
In terms of application, the Europe electronic patient-reported outcomes (ePROS) market is segmented into oncology, respiratory, and others. The oncology segment held the largest share of the Europe electronic patient-reported outcomes (ePROS) market share in 2023.
By end user, the Europe electronic patient-reported outcomes (ePROS) market is segmented into contract research organizations (CROs), pharmaceutical companies, and others. The pharmaceutical companies segment held the largest share of the Europe electronic patient-reported outcomes (ePROS) market share in 2023.
Based on country, the Europe electronic patient-reported outcomes (ePROS) market is segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe. Germany segment held the largest share of Europe electronic patient-reported outcomes (ePROS) market in 2023.
Assistek, Buddy Healthcare Ltd Oy, Castor, Clinical Ink Inc, Crucial Data Solutions, Curebase, Medable Inc, Medidata Solutions, Medrio, OpenClinica LLC, PatientIQ, Signant Health, Veeva Systems Inc, and Y-Prime LLC are some of the leading companies operating in the Europe electronic patient-reported outcomes (ePROS) market.
The Europe Electronic Patient-Reported Outcomes (ePROS) Market is valued at US$ 268.32 Million in 2023, it is projected to reach US$ 889.08 Million by 2031.
As per our report Europe Electronic Patient-Reported Outcomes (ePROS) Market, the market size is valued at US$ 268.32 Million in 2023, projecting it to reach US$ 889.08 Million by 2031. This translates to a CAGR of approximately 16.2% during the forecast period.
The Europe Electronic Patient-Reported Outcomes (ePROS) Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Electronic Patient-Reported Outcomes (ePROS) Market report:
The Europe Electronic Patient-Reported Outcomes (ePROS) Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Electronic Patient-Reported Outcomes (ePROS) Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Electronic Patient-Reported Outcomes (ePROS) Market value chain can benefit from the information contained in a comprehensive market report.