Colorectal cancer is a malignant tumor that develops in colonic or rectal tissues. As per the data provided by the European Cancer Information System, in EU-27 countries, colorectal cancer accounted for 12.7% of all new cancer diagnoses and 12.4% of total mortalities caused by the disease in 2020. Such a high prevalence makes it the second most frequently occurring cancer, after breast cancer, and the second leading cause of cancer-related deaths, after lung cancer. Surgery is the most common treatment for all stages of colon cancer. In ideal situations, if the cancer is diagnosed in the early stages, doctors can remove the tumor via surgical procedures. A colonoscopy is an important screening test for colorectal cancer diagnostics, and it has become a part of routine cancer screening. Thus, rising prevalence of colorectal cancer drives the growth of the Europe colorectal cancer diagnostics market.
The European colorectal cancer diagnostics market is segmented into Germany, the UK, France, Italy, Spain, and the Rest of Europe. In Germany, the colorectal cancer diagnostics market is expected to grow in the coming years due to the rising development in the field of cancer and increasing prevalence of cancer. The National Cancer Plan of the Federal Ministry of Health (Nationaler Krebsplan des Bundesministeriums für Gesundheit) paved the way for the law to develop the cancer detection and quality assurance clinical cancer Registration Act (Gesetz zur Weiterentwicklung der Krebsfrüherkennung und zur Qualitätssicherung durch klinische Krebsregister), which the German parliament approved in 2013. This law prescribes the implementation of organized and quality-assured colon cancer screening programs. In addition, the screening approach for colorectal cancer in the country offers both men and women at least five annual faecal immunochemical tests (FITs) at ages 50–54, followed by a first screening colonoscopy at 55 years in case all of the FITs were negative. On April 4, 2019, a colorectal screening program was organized on the national level, which requires men aged 50 years and women aged 55 years of age to undergo a colonoscopy because of the rise in colorectal cancer risk. The Federal Joint Committee (G-BA) adopted the directive on organized cancer screening programs (oKFE-RL). Colorectal cancer screening was the first organized program to be implemented after that, and starting from July 2019, insured people aged 50 years are regularly invited for screening by health insurance companies. Therefore, the growing prevalence of colorectal cancer and increasing government approaches toward colorectal cancer screening are among the factors driving the Europe colorectal cancer diagnostics market growth.
Strategic insights for the Europe Colorectal Cancer Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Europe Colorectal Cancer Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.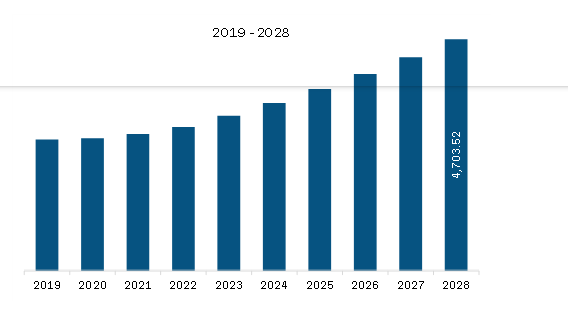
Europe Colorectal Cancer Diagnostics Strategic Insights
Europe Colorectal Cancer Diagnostics Report Scope
Report Attribute
Details
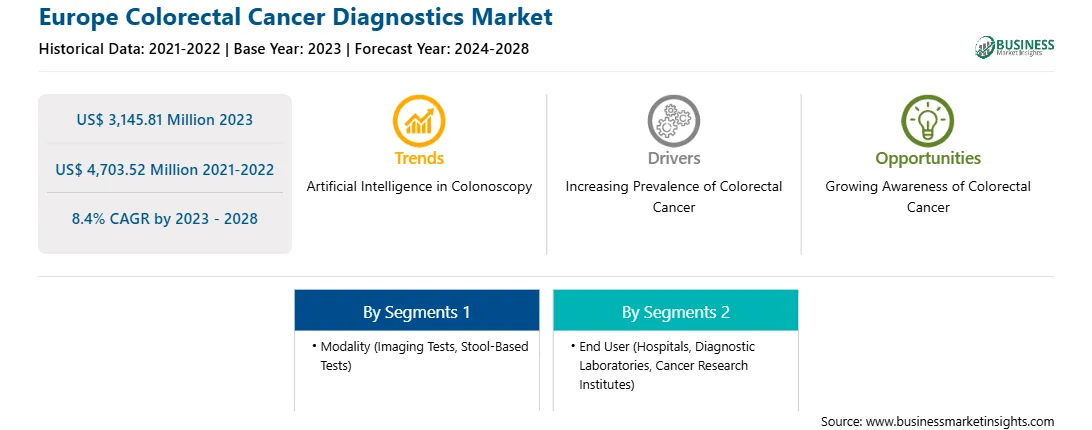
Market size in 2023
US$ 3,145.81 Million
Market Size by 2028
US$ 4,703.52 Million
Global CAGR (2023 - 2028)
8.4%
Historical Data
2021-2022
Forecast period
2024-2028
Segments Covered
By Modality
By End User
Regions and Countries Covered
Europe
Market leaders and key company profiles
Europe Colorectal Cancer Diagnostics Regional Insights
Europe Colorectal Cancer Diagnostics Market Segmentation
The Europe colorectal cancer diagnostics market is segmented into modality, end user, and country.
Based on modality, the Europe colorectal cancer diagnostics market is bifurcated into imaging tests and stool-based tests. In 2023, the imaging tests segment held a larger share of the Europe colorectal cancer diagnostics market. The market for the imaging tests segment is further segmented into colonoscopy, CT colonography, flexible sigmoidoscopy, capsule endoscopy, and others. The market for the stool based tests segment is subsegmented into faecal immunochemical test (fit), guaiac-based faecal occult blood test (gFOBT), and stool DNA test.
Based on end user, the Europe colorectal cancer diagnostics market is segmented into hospitals, diagnostic laboratories, cancer research institutes, and others. In 2023, the hospitals segment held the largest share of the Europe colorectal cancer diagnostics market.
Based on country, the Europe colorectal cancer diagnostics market is segmented into France, Germany, the UK, Italy, Spain, and the Rest of Europe. In 2023, Germany accounted for the largest share of the Europe colorectal cancer diagnostics market.
Medtronic Plc, Illumina Inc, Epigenomics AG, Novigenix SA, F. Hoffmann-La Roche Ltd, Quest Diagnostics Inc, Siemens Healthineers AG, Bruker Corp, and Eiken Chemical Co., Ltd. are the leading companies operating in the Europe colorectal cancer diagnostics market.
The Europe Colorectal Cancer Diagnostics Market is valued at US$ 3,145.81 Million in 2023, it is projected to reach US$ 4,703.52 Million by 2028.
As per our report Europe Colorectal Cancer Diagnostics Market, the market size is valued at US$ 3,145.81 Million in 2023, projecting it to reach US$ 4,703.52 Million by 2028. This translates to a CAGR of approximately 8.4% during the forecast period.
The Europe Colorectal Cancer Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Colorectal Cancer Diagnostics Market report:
The Europe Colorectal Cancer Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Colorectal Cancer Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Colorectal Cancer Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.