The ultimate goal of drug development is to bring new compounds with proven therapeutic effects to the market. Obtaining accurate information on conducted trials' outcomes poses a significant challenge in estimating the success rate of clinical trials, as collecting such data is expensive, time-consuming, and prone to errors. In-vivo imaging performed on human subjects in clinical trials are likely to improve the success rate of the drug development process. For example, positron emission tomography (PET) imaging can visualize radio-labeled compounds that are intended for targeted drugs in-vivo activities or monitor compound tissue exposure during clinical trial testing. Other in-vivo imaging techniques, such as magnetic resonance imaging (MRI), efficiently visualize human anatomy with the capability to visualize drug molecules in the body of clinical trial participants capable of detecting adequate sensitivity and specificity. Mass spectrometry imaging (MSI) is currently utilized to visualize drug molecules for ex-vivo tissue sections. Therefore, the increasing use of imaging techniques or modalities for clinical trial testing to determine clinical dose efficacy and toxicity leads to greater success rates of clinical trials, which, in turn, is favoring the growth of the Europe clinical trial imaging market.
The Europe clinical trial imaging market is segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe. Rise in the number of clinical trial activities for new drug development and innovative product launches by the service provider companies are a few factors likely to support the market growth in Europe during the forecast period. Further, cancer, cardiovascular illness, nervous system disorders, and infectious diseases are the most frequently studied conditions in clinical trials in Germany. Clinical research benefits from Germany's well-established infrastructure for transport, communication, energy, and public services, along with the large patient pool and high demand for quality healthcare. Coordinating centers for clinical trials were established as part of a new funding program under the Federal Ministry of Education and Research to strengthen academic clinical research. Therefore, Germany's clinical trial approval processes are standardized, reliable, transparent, and approved for relatively short study start-up timelines. Further, in Germany, top CROs provide clinical trial imaging software for imaging studies. Pharmtrace, a Berlin-based CRO's "ERICA," provides a flexible platform for clinical trial image management combining regulatory compliance for the collection, centralization, and QC of clinical trial medical images. ERICA was developed and validated by pharmtrace intended for real-life clinical trials to document and make clinical imaging GCP-compliant correctly.
Strategic insights for the Europe Clinical Trial Imaging provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Europe Clinical Trial Imaging refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Europe Clinical Trial Imaging Strategic Insights
Europe Clinical Trial Imaging Report Scope
Report Attribute
Details
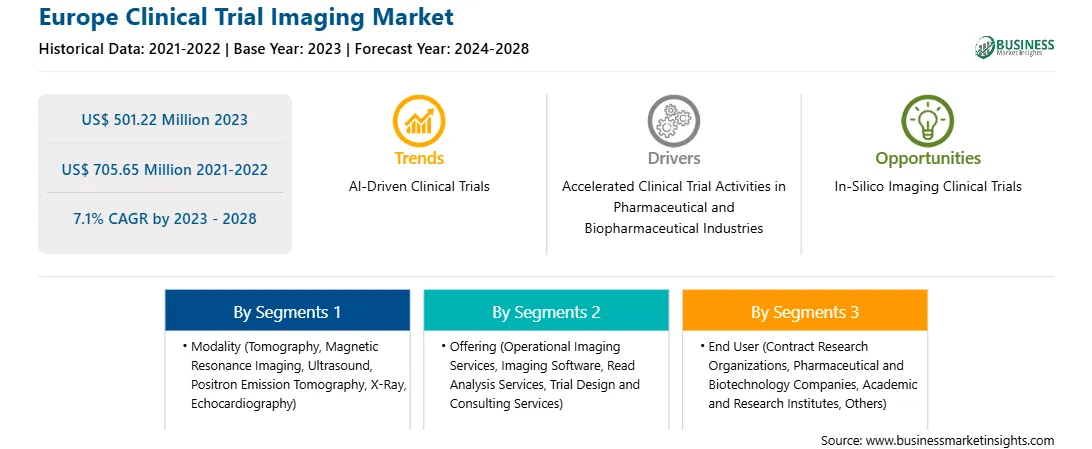
Market size in 2023
US$ 501.22 Million
Market Size by 2028
US$ 705.65 Million
Global CAGR (2023 - 2028)
7.1%
Historical Data
2021-2022
Forecast period
2024-2028
Segments Covered
By Modality
By Offering
By End User
Regions and Countries Covered
Europe
Market leaders and key company profiles
Europe Clinical Trial Imaging Regional Insights
Europe Clinical Trial Imaging Market Segmentation
The Europe clinical trial imaging market is segmented on the basis of modality, offering, end user, and country.
Based on modality, the Europe clinical trial imaging market is segmented into tomography, magnetic resonance imaging (MRI), ultrasound, positron emission tomography (PET), X-ray, echocardiography, and others. The tomography segment registered the largest market share in 2023.
Based on offering, the Europe clinical trial imaging market is segmented into operational imaging services, imaging software, read analysis services, trial design and consulting services, and others. The operational imaging services segment held the largest market share in 2023.
Based on end user, the Europe clinical trial imaging market is segmented into contract research organizations (CROs), pharmaceutical and biotechnology companies, academic and research institutes, and others. The contract research organizations (CROs) segment held the largest market share in 2023.
Based on country, the market is segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe. Germany dominated the market share in 2023.
Calyx Inc, eResearch Technology Inc, ICON PLC, IXICO plc, Medical Metrics Inc, VIDA Diagnostics Inc, and WCG Clinical Inc are the leading companies operating in the Europe clinical trial imaging market.
The Europe Clinical Trial Imaging Market is valued at US$ 501.22 Million in 2023, it is projected to reach US$ 705.65 Million by 2028.
As per our report Europe Clinical Trial Imaging Market, the market size is valued at US$ 501.22 Million in 2023, projecting it to reach US$ 705.65 Million by 2028. This translates to a CAGR of approximately 7.1% during the forecast period.
The Europe Clinical Trial Imaging Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Clinical Trial Imaging Market report:
The Europe Clinical Trial Imaging Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Clinical Trial Imaging Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Clinical Trial Imaging Market value chain can benefit from the information contained in a comprehensive market report.