Due to advancements in technologies related to feasibility and processing times, circulating tumor cell diagnostics and liquid biopsy have recently gathered considerable interest as a noninvasive alternative to tissue biopsy in cancer patients. The implementation of liquid biopsy procedures is supported by a greater understanding of improved minimally invasive cancer detection technologies. The market growth will be influenced by the rising demand for noninvasive tools, and laboratory acceptance of CTCs tests and liquid biopsy. A liquid biopsy allows doctors to obtain information about tumors from a simple blood sample. A blood test is painless, noninvasive, cost-effective, and less time-consuming. In addition, CTCs, cfDNAs, exosomes, and microvesicles can be detected in a blood sample, making blood-based liquid biopsies more popular.
The COVID-19 emergency has encouraged the general population to try out digital services and scale up their usage, particularly to avoid having to visit a health center in person. The unprecedented impact of COVID-19 has been far-reaching, but the effects on cancer patients make them one of the worst affected groups. COVID-19 has profoundly impacted the number of patients undergoing cancer screening, diagnosis, and treatment. The increased pressure due to the growing rate of COVID-19 patients' hospitalization led to the re-profiling of many hospitals and departments, including oncology clinics, for accommodating the increasingly large number of COVID-19 patients. According to one study, many diagnostic and treatment procedures, including 2.3 million cancer surgeries, were canceled or postponed worldwide. Thus, the pandemic hindered the circulating tumor cell (CTC) diagnostics market growth.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the circulating tumor cell (CTC) diagnostics market. The Europe circulating tumor cell (CTC) diagnostics market is expected to grow at a good CAGR during the forecast period.
Europe Circulating Tumor Cell (CTC) Diagnostics Market Segmentation
By Technology
Strategic insights for the Europe Circulating Tumor Cell (CTC) Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
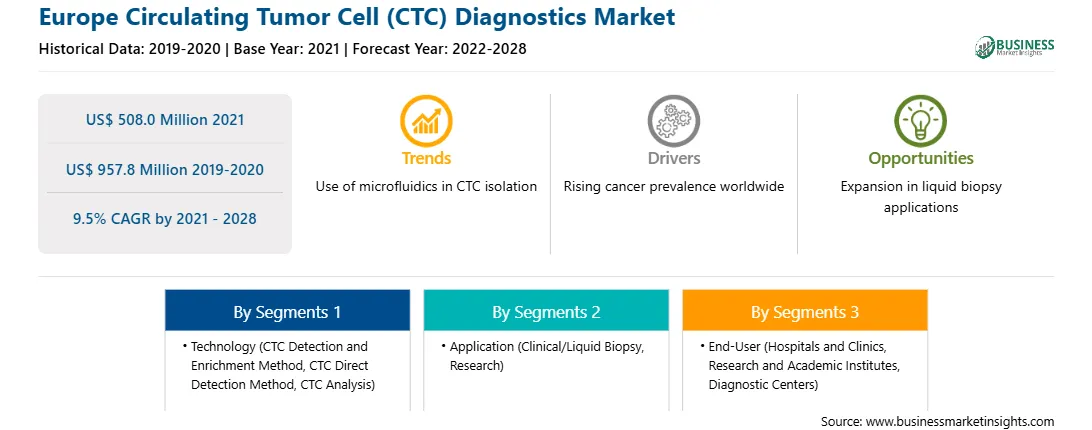
| Market size in 2021 | US$ 508.0 Million |
| Market Size by 2028 | US$ 957.8 Million |
| Global CAGR (2021 - 2028) | 9.5% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Technology
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Circulating Tumor Cell (CTC) Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
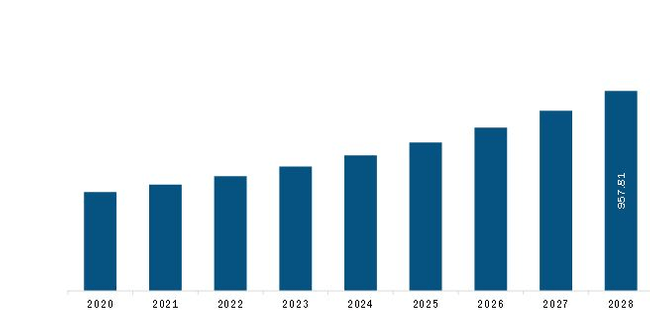
The Europe Circulating Tumor Cell (CTC) Diagnostics Market is valued at US$ 508.0 Million in 2021, it is projected to reach US$ 957.8 Million by 2028.
As per our report Europe Circulating Tumor Cell (CTC) Diagnostics Market, the market size is valued at US$ 508.0 Million in 2021, projecting it to reach US$ 957.8 Million by 2028. This translates to a CAGR of approximately 9.5% during the forecast period.
The Europe Circulating Tumor Cell (CTC) Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Circulating Tumor Cell (CTC) Diagnostics Market report:
The Europe Circulating Tumor Cell (CTC) Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Circulating Tumor Cell (CTC) Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Circulating Tumor Cell (CTC) Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.